Longitudinal shortening remains the principal component of left ventricular pumping in patients with chronic myocardial infarction even when the absolute atrioventricular plane displacement is decreased
- PMID: 28754098
- PMCID: PMC5534092
- DOI: 10.1186/s12872-017-0641-z
Longitudinal shortening remains the principal component of left ventricular pumping in patients with chronic myocardial infarction even when the absolute atrioventricular plane displacement is decreased
Abstract
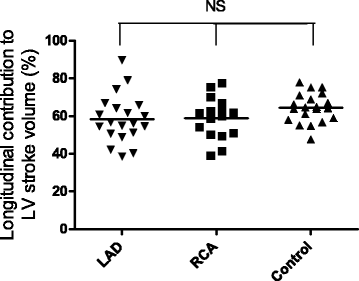
Background: The majority (60%) of left ventricular (LV) stroke volume (SV) is generated by longitudinal shortening causing apical atrioventricular plane displacement (AVPD) in systole. The remaining SV is caused by radial inward motion of the epicardium both in the septal and the lateral wall. We aimed to determine if these longitudinal, septal and lateral contributions to LVSV are changed in patients with chronic myocardial infarction (MI).
Methods: Patients with a chronic (>3 months) ST-elevation MI in the left anterior descending (LAD, n = 20) or right coronary artery (RCA, n = 16) and healthy controls (n = 20) were examined with cardiovascular magnetic resonance (CMR). AVPD was quantified in long axis cine CMR images and LV volumes and dimensions in short axis cine images.
Results: AVPD was decreased both in patients with LAD-MI (11 ± 1 mm, p < 0.001) and RCA-MI (13 ± 1 mm, p < 0.05) compared to controls (15 ± 0 mm). However, the longitudinal contribution to SV was unchanged for both LAD-MI (58 ± 3%, p = 0.08) and RCA-MI (59 ± 3%, p = 0.09) compared to controls (64 ± 2%). The preserved longitudinal contribution despite decreased absolute AVPD was a results of increased epicardial dimensions (p < 0.01 for LAD-MI and p = 0.06 for RCA-MI). In LAD-MI the septal contribution to LVSV was decreased (5 ± 1%) compared to both controls (10 ± 1%, p < 0.01) and patients with RCA-MIs (10 ± 1%, p < 0.01). The lateral contribution was increased in LAD-MI patients (44 ± 3%) compared to both RCA-MI (35 ± 2%, p < 0.05) and controls (29 ± 2%, p < 0.001).
Conclusion: Longitudinal shortening remains the principal component of left ventricular pumping in patients with chronic MI even when the absolute AVPD is decreased.
Keywords: Cardiac output; Cardiac pumping; Heart failure; Late gadolinium enhancement; Mitral annular plane systolic excursion; Regional function.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was granted by Regional ethical committee in Lund and written informed consent to participate was acquired.
Consent for publication
Consent for publication was granted in conjunction with the ethics approval and consent to participate in the study. Images are anonymized and without possibility to track to individual patients from the manuscript.
Competing interests
MC and HE have received consultancy fees from Imacor AB and HA is shoreholder in Imacor AB performing CMR analysis in multicenter trials.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






Similar articles
-
Evolution of left ventricular function among subjects with ST-elevation myocardial infarction after percutaneous coronary intervention.BMC Cardiovasc Disord. 2020 Jun 29;20(1):309. doi: 10.1186/s12872-020-01540-y. BMC Cardiovasc Disord. 2020. PMID: 32600336 Free PMC article. Clinical Trial.
-
Time-resolved tracking of the atrioventricular plane displacement in Cardiovascular Magnetic Resonance (CMR) images.BMC Med Imaging. 2017 Feb 28;17(1):19. doi: 10.1186/s12880-017-0189-5. BMC Med Imaging. 2017. PMID: 28241751 Free PMC article.
-
Regional contribution to ventricular stroke volume is affected on the left side, but not on the right in patients with pulmonary hypertension.Int J Cardiovasc Imaging. 2016 Aug;32(8):1243-53. doi: 10.1007/s10554-016-0898-9. Epub 2016 May 3. Int J Cardiovasc Imaging. 2016. PMID: 27142431
-
Longitudinal left ventricular function is globally depressed within a week of STEMI.Clin Physiol Funct Imaging. 2018 Apr 27. doi: 10.1111/cpf.12521. Online ahead of print. Clin Physiol Funct Imaging. 2018. PMID: 29701310
-
Myocardial bridge as a structure of "double-edged sword" for the coronary artery.Ann Vasc Dis. 2014;7(2):99-108. doi: 10.3400/avd.ra.14-00037. Epub 2014 May 16. Ann Vasc Dis. 2014. PMID: 24995053 Free PMC article. Review.
Cited by
-
Reference Values for Inward Displacement in the Normal Left Ventricle: A Novel Method of Regional Left Ventricular Function Assessment.J Cardiovasc Dev Dis. 2023 Nov 24;10(12):474. doi: 10.3390/jcdd10120474. J Cardiovasc Dev Dis. 2023. PMID: 38132642 Free PMC article.
-
Cardiac magnetic resonance-derived mitral annular plane systolic excursion: a robust indicator for risk stratification after myocardial infarction.Int J Cardiovasc Imaging. 2024 Apr;40(4):897-906. doi: 10.1007/s10554-024-03058-2. Epub 2024 Feb 24. Int J Cardiovasc Imaging. 2024. PMID: 38400864
-
Longitudinal Displacement for Left Ventricular Function Assessment.J Cardiovasc Dev Dis. 2025 Jan 31;12(2):53. doi: 10.3390/jcdd12020053. J Cardiovasc Dev Dis. 2025. PMID: 39997487 Free PMC article.
-
Anterior STEMI associated with decreased strain in remote cardiac myocardium.Int J Cardiovasc Imaging. 2022 Feb;38(2):375-387. doi: 10.1007/s10554-021-02391-0. Epub 2021 Sep 5. Int J Cardiovasc Imaging. 2022. PMID: 34482507 Free PMC article. Clinical Trial.
-
MRI-Based Circumferential Strain in Boys with Early Duchenne Muscular Dystrophy Cardiomyopathy.Diagnostics (Basel). 2024 Nov 27;14(23):2673. doi: 10.3390/diagnostics14232673. Diagnostics (Basel). 2024. PMID: 39682580 Free PMC article.
References
-
- McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur J Heart Fail. 2012;14(8):803–869. doi: 10.1093/eurjhf/hfs105. - DOI - PubMed
-
- Palazzuoli A, Beltrami M, Gennari L, Dastidar AG, Nuti R, McAlindon E, Angelini GD, Bucciarelli-Ducci C. The impact of infarct size on regional and global left ventricular systolic function: a cardiac magnetic resonance imaging study. The international journal of cardiovascular imaging. 2015;31(5):1037–1044. doi: 10.1007/s10554-015-0657-3. - DOI - PubMed
-
- Rubenstein JC, Ortiz JT, Wu E, Kadish A, Passman R, Bonow RO, Goldberger JJ. The use of periinfarct contrast-enhanced cardiac magnetic resonance imaging for the prediction of late postmyocardial infarction ventricular dysfunction. Am Heart J. 2008;156(3):498–505. doi: 10.1016/j.ahj.2008.04.012. - DOI - PubMed
-
- van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, et al. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovasc Imaging. 2014;7(9):930–939. doi: 10.1016/j.jcmg.2014.05.010. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

