Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis
- PMID: 28754611
- PMCID: PMC5736097
- DOI: 10.1016/j.jconrel.2017.07.032
Transdermal delivery of gentamicin using dissolving microneedle arrays for potential treatment of neonatal sepsis
Abstract
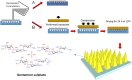
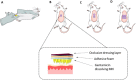
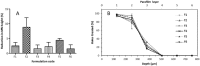
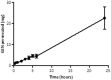
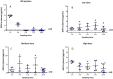
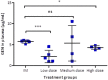
Neonatal infections are a leading cause of childhood mortality in low-resource settings. World Health Organization guidelines for outpatient treatment of possible serious bacterial infection (PSBI) in neonates and young infants when referral for hospital treatment is not feasible include intramuscular gentamicin (GEN) and oral amoxicillin. GEN is supplied as an aqueous solution of gentamicin sulphate in vials or ampoules and requires health care workers to be trained in dose calculation or selection of an appropriate dose based on the patient's weight band and to have access to safe injection supplies and appropriate sharps disposal. A simplified formulation, packaging, and delivery method to treat PSBI in low-resource settings could decrease user error and expand access to lifesaving outpatient antibiotic treatment for infants with severe infection during the neonatal period. We developed dissolving polymeric microneedles (MN) arrays to deliver GEN transdermally. MN arrays were produced from aqueous blends containing 30% (w/w) of GEN and two polymers approved by the US Food and Drug Administration: sodium hyaluronate and poly(vinylpyrrolidone). The arrays (19×19 needles and 500μm height) were mechanically strong and were able to penetrate a skin simulant to a depth of 378μm. The MN arrays were tested in vitro using a Franz Cell setup delivering approximately 4.45mg of GEN over 6h. Finally, three different doses (low, medium, and high) of GEN delivered by MN arrays were tested in an animal model. Maximum plasma levels of GEN were dose-dependent and ranged between 2 and 5μg/mL. The time required to reach these levels post-MN array application ranged between 1 and 6h. This work demonstrated the potential of dissolving MN arrays to deliver GEN transdermally at therapeutic levels in vivo.
Keywords: Gentamicin; Microneedle; Neonatal sepsis.
Copyright © 2017 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Rapidly dissolving bilayer microneedle arrays - A minimally invasive transdermal drug delivery system for vitamin B12.Int J Pharm. 2019 Jul 20;566:299-306. doi: 10.1016/j.ijpharm.2019.05.066. Epub 2019 May 28. Int J Pharm. 2019. PMID: 31150773
-
Fabrication and mechanical/biological evaluations of dissolving bird-bill microneedle arrays.Drug Deliv Transl Res. 2025 Jul;15(7):2581-2588. doi: 10.1007/s13346-024-01757-w. Epub 2024 Dec 9. Drug Deliv Transl Res. 2025. PMID: 39653959 Free PMC article.
-
Dissolving polymeric microneedle arrays for enhanced site-specific acyclovir delivery.Eur J Pharm Sci. 2018 Aug 30;121:200-209. doi: 10.1016/j.ejps.2018.05.009. Epub 2018 May 16. Eur J Pharm Sci. 2018. PMID: 29777854
-
Microneedles-Based Transdermal Drug Delivery Systems: A Review.J Biomed Nanotechnol. 2017 Dec 1;13(12):1581-1597. doi: 10.1166/jbn.2017.2474. J Biomed Nanotechnol. 2017. PMID: 29490749 Review.
-
Microneedle arrays as medical devices for enhanced transdermal drug delivery.Expert Rev Med Devices. 2011 Jul;8(4):459-82. doi: 10.1586/erd.11.20. Expert Rev Med Devices. 2011. PMID: 21728732 Review.
Cited by
-
Polymeric Microneedles for Transdermal Delivery of Rivastigmine: Design and Application in Skin Mimetic Model.Pharmaceutics. 2022 Mar 30;14(4):752. doi: 10.3390/pharmaceutics14040752. Pharmaceutics. 2022. PMID: 35456586 Free PMC article.
-
Pullulan-based dissolving microneedle arrays for enhanced transdermal delivery of small and large biomolecules.Int J Biol Macromol. 2020 Mar 1;146:290-298. doi: 10.1016/j.ijbiomac.2019.12.184. Epub 2019 Dec 26. Int J Biol Macromol. 2020. PMID: 31883883 Free PMC article.
-
Rethinking antimicrobial stewardship paradigms in the context of the gut microbiome.JAC Antimicrob Resist. 2019 May 21;1(1):dlz015. doi: 10.1093/jacamr/dlz015. eCollection 2019 Jun. JAC Antimicrob Resist. 2019. PMID: 34222889 Free PMC article. Review.
-
Design, formulation and evaluation of novel dissolving microarray patches containing a long-acting rilpivirine nanosuspension.J Control Release. 2018 Dec 28;292:119-129. doi: 10.1016/j.jconrel.2018.11.002. Epub 2018 Nov 2. J Control Release. 2018. PMID: 30395897 Free PMC article.
-
Hybrid microneedle arrays for antibiotic and near-IR photothermal synergistic antimicrobial effect against Methicillin-Resistant Staphylococcus aureus.Chem Eng J. 2023 Apr;462:142127. doi: 10.1016/j.cej.2023.142127. Chem Eng J. 2023. PMID: 37719675 Free PMC article.
References
-
- Lawn J.E., Cousens S., Zupan J. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. - PubMed
-
- Liu L., Oza S., Hogan D., Perin J., Rudan I., Lawn J.E., Cousens S., Mathers C., Black R.E. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. - PubMed
-
- World Health Organization . 2015. GUIDELINE Managing Possible Serious Bacterial Infection in Young Infants When Referral Is Not Feasible. - PubMed
-
- Šoltés L. Aminoglycoside antibiotics — two decades of their HPLC bioanalysis. Biomed. Chromatogr. 1999;13:3–10. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

