Nitric Oxide Modulates Macrophage Responses to Mycobacterium tuberculosis Infection through Activation of HIF-1α and Repression of NF-κB
- PMID: 28754681
- PMCID: PMC5568107
- DOI: 10.4049/jimmunol.1700515
Nitric Oxide Modulates Macrophage Responses to Mycobacterium tuberculosis Infection through Activation of HIF-1α and Repression of NF-κB
Abstract
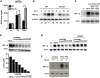
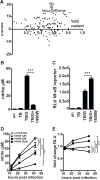
IFN-γ is essential for control of Mycobacterium tuberculosis infection in vitro and in vivo. However, the mechanisms by which IFN-γ controls infection remain only partially understood. One of the crucial IFN-γ target genes required for control of M. tuberculosis is inducible NO synthase (iNOS). Although NO produced by iNOS is thought to have direct bactericidal activity against M. tuberculosis, the role of NO as a signaling molecule has been poorly characterized in the context M. tuberculosis infection. In this study, we found that iNOS broadly regulates the macrophage transcriptome during M. tuberculosis infection, activating antimicrobial pathways while also limiting inflammatory cytokine production. The transcription factor hypoxia inducible factor-1α (HIF-1α) was recently shown to be critical for IFN-γ-mediated control of M. tuberculosis infection. We found that HIF-1α function requires NO production, and that HIF-1α and iNOS are linked by a positive feedback loop that amplifies macrophage activation. Furthermore, we found that NO inhibits NF-κB activity to prevent hyperinflammatory responses. Thus, NO activates robust microbicidal programs while also limiting damaging inflammation. IFN-γ signaling must carefully calibrate an effective immune response that does not cause excessive tissue damage, and this study identifies NO as a key player in establishing this balance during M. tuberculosis infection.
Copyright © 2017 by The American Association of Immunologists, Inc.
Figures
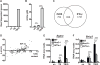
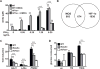


References
-
- Floyd K. World Health Organizationed. WHO Press; 2016. pp. 1–100.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

