Regulation of Nephron Progenitor Cell Self-Renewal by Intermediary Metabolism
- PMID: 28754792
- PMCID: PMC5661282
- DOI: 10.1681/ASN.2016111246
Regulation of Nephron Progenitor Cell Self-Renewal by Intermediary Metabolism
Abstract
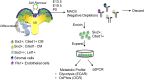
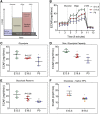
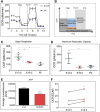
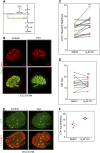
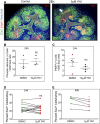
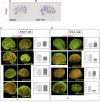
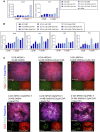
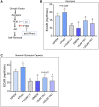
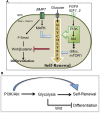
Nephron progenitor cells (NPCs) show an age-dependent capacity to balance self-renewal with differentiation. Older NPCs (postnatal day 0) exit the progenitor niche at a higher rate than younger (embryonic day 13.5) NPCs do. This behavior is reflected in the transcript profiles of young and old NPCs. Bioenergetic pathways have emerged as important regulators of stem cell fate. Here, we investigated the mechanisms underlying this regulation in murine NPCs. Upon isolation and culture in NPC renewal medium, younger NPCs displayed a higher glycolysis rate than older NPCs. Inhibition of glycolysis enhanced nephrogenesis in cultured embryonic kidneys, without increasing ureteric tree branching, and promoted mesenchymal-to-epithelial transition in cultured isolated metanephric mesenchyme. Cotreatment with a canonical Wnt signaling inhibitor attenuated but did not entirely block the increase in nephrogenesis observed after glycolysis inhibition. Furthermore, inhibition of the phosphatidylinositol 3-kinase/Akt self-renewal signaling pathway or stimulation of differentiation pathways in the NPC decreased glycolytic flux. Our findings suggest that glycolysis is a pivotal, cell-intrinsic determinant of NPC fate, with a high glycolytic flux supporting self-renewal and inhibition of glycolysis stimulating differentiation.
Keywords: Cell Signaling; Differentiation; Glycolysis; PI3K/Akt; Stem Cell Renewal; kidney development.
Copyright © 2017 by the American Society of Nephrology.
Figures
Comment in
-
New Insights into Fuel Choices of Nephron Progenitor Cells.J Am Soc Nephrol. 2017 Nov;28(11):3133-3135. doi: 10.1681/ASN.2017070795. Epub 2017 Sep 5. J Am Soc Nephrol. 2017. PMID: 28874403 Free PMC article. No abstract available.
References
-
- Hoy WEH, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF: Reduced nephron number and glomerulomegaly in Australian Aborigines: A group at high risk for renal disease and hypertension. Kidney Int 70: 104–110, 2006 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

