Transcriptomic signature of the follicular somatic compartment surrounding an oocyte with high developmental competence
- PMID: 28755009
- PMCID: PMC5533789
- DOI: 10.1038/s41598-017-07039-5
Transcriptomic signature of the follicular somatic compartment surrounding an oocyte with high developmental competence
Abstract
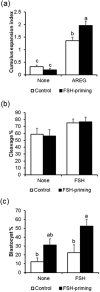
During antral folliculogenesis, developmental competence of prospective oocytes is regulated in large part by the follicular somatic component to prepare the oocyte for the final stage of maturation and subsequent embryo development. The underlying molecular mechanisms are poorly understood. Oocytes reaching the advanced stage of follicular growth by administration of exogenous follicle-stimulating hormone (FSH) possess higher developmental competence than oocytes in FSH-untreated smaller follicles. In this study, the transcriptomic profile of the cumulus cells from cows receiving FSH administration (FSH-priming) was compared, as a model of high oocyte competence, with that from untreated donor cows (control). Ingenuity Pathway Analysis showed that cumulus cells receiving FSH-priming were rich in down-regulated transcripts associated with cell movement and migration, including the extracellular matrix-related transcripts, probably preventing the disruption of cell-to-cell contacts. Interestingly, the transcriptomic profile of up-regulated genes in the control group was similar to that of granulosa cells from atretic follicles. Interferon regulatory factor 7 was activated as the key upstream regulator of FSH-priming. Thus, acquisition of developmental competence by oocytes can be ensured by the integrity of cumulus cells involved in cell-to-cell communication and cell survival, which may help achieve enhanced oocyte-somatic cell coupling.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




Similar articles
-
Differences in cumulus cell gene expression indicate the benefit of a pre-maturation step to improve in-vitro bovine embryo production.Mol Hum Reprod. 2016 Dec;22(12):882-897. doi: 10.1093/molehr/gaw055. Epub 2016 Aug 24. Mol Hum Reprod. 2016. PMID: 27559149
-
Characterization of FSH signalling networks in bovine cumulus cells: a perspective on oocyte competence acquisition.Mol Hum Reprod. 2015 Sep;21(9):688-701. doi: 10.1093/molehr/gav032. Epub 2015 Jun 24. Mol Hum Reprod. 2015. PMID: 26113519
-
Functional signaling and gene regulatory networks between the oocyte and the surrounding cumulus cells.BMC Genomics. 2018 May 10;19(1):351. doi: 10.1186/s12864-018-4738-2. BMC Genomics. 2018. PMID: 29747587 Free PMC article.
-
Somatic environment and germinal differentiation in antral follicle: The effect of FSH withdrawal and basal LH on oocyte competence acquisition in cattle.Theriogenology. 2016 Jul 1;86(1):54-61. doi: 10.1016/j.theriogenology.2016.04.018. Epub 2016 Apr 21. Theriogenology. 2016. PMID: 27158126 Review.
-
The epidermal growth factor network: role in oocyte growth, maturation and developmental competence.Hum Reprod Update. 2018 Jan 1;24(1):1-14. doi: 10.1093/humupd/dmx029. Hum Reprod Update. 2018. PMID: 29029246 Review.
Cited by
-
Factors affecting the in vitro embryo production in buffalo (Bubalus bubalis): A review.Vet Med (Praha). 2023 Feb 21;68(2):45-56. doi: 10.17221/48/2022-VETMED. eCollection 2023 Feb. Vet Med (Praha). 2023. PMID: 38332761 Free PMC article. Review.
-
Follicular guidance for oocyte developmental competence.Anim Reprod. 2018 Aug 3;15(Suppl 1):721-725. doi: 10.21451/1984-3143-AR2018-0035. eCollection 2018 Jul-Sep. Anim Reprod. 2018. PMID: 36249831 Free PMC article.
-
DENND1A desensitizes granulosa cells to FSH by arresting intracellular FSHR transportation.Sci China Life Sci. 2024 Aug;67(8):1620-1634. doi: 10.1007/s11427-023-2438-4. Epub 2024 Apr 23. Sci China Life Sci. 2024. PMID: 38709439
-
Protease expression in the human and rat cumulus-oocyte complex during the periovulatory period: a role in cumulus-oocyte complex migration†.Biol Reprod. 2024 Oct 14;111(4):845-855. doi: 10.1093/biolre/ioae108. Biol Reprod. 2024. PMID: 39018235 Free PMC article.
-
Neuregulin-1 signaling regulates cytokines and chemokines expression and secretion in granulosa cell.J Ovarian Res. 2022 Jul 26;15(1):86. doi: 10.1186/s13048-022-01021-0. J Ovarian Res. 2022. PMID: 35883098 Free PMC article.
References
-
- El-Hayek S, Demeestere I, Clarke HJ. Follicle-stimulating hormone regulates expression and activity of epidermal growth factor receptor in the murine ovarian follicle. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16778–16783. doi: 10.1073/pnas.1414648111. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

