Adjuvant endocrine monotherapy for postmenopausal early breast cancer patients with hormone-receptor positive: a systemic review and network meta-analysis
- PMID: 28755088
- PMCID: PMC5741789
- DOI: 10.1007/s12282-017-0794-8
Adjuvant endocrine monotherapy for postmenopausal early breast cancer patients with hormone-receptor positive: a systemic review and network meta-analysis
Abstract
Background: In patients with hormone receptor-positive postmenopausal of early stage breast cancer, adjuvant endocrine monotherapies include letrozole, anastrozole, exemestane, toremifene and tamoxifen. But the optimum regimen remains controversial.
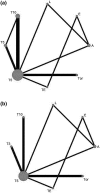
Methods: PubMed, Cochrane Database and ClinicalTrials.gov were systematically reviewed of abstract for randomized-controlled trials (RCTs) to assess the efficacy of tamoxifen, letrozole, exemestane, anastrozle and toremifene for postmenopausal patients with hormone-receptor positive (HR+), who have not received prior therapy for early stage breast cancer. The outcomes were measured by disease-free survival (DFS) and overall survival (OS). We evaluated relative hazard ratios (HRs) for death of different therapies by combination hazard ratios for death of included trials. The SUCRA values were used to evaluate the rankings of efficacy for these monotherapies.
Results: A total of fourteen studies including 19,517 patients in our research were absorbed and estimated. The superiority of efficacy for DFS were 5-year letrozole and 10-year tamoxifen (SUCRA values 0.743/0.657) in all comparisons. A more efficient SUCRA values for OS were 5-year Exemestane, 5-year letrozole and 10-year tamoxifen (0.756/0.677/0.669).
Conclusions: Clinically important differences exist between commonly prescribed different adjuvant endocrine monotherapy regimens for both efficacy and acceptability in favor of exemestane and letrozole. 10-year tamoxifen for early breast cancer patients is noninferior to 5-year anastrozle, and might be the best choice where aromatase inhibitors (AIs) are not easy to acquire.
Keywords: Early breast cancer; Endocrine monotherapy; Network meta-analysis; Postmenopausal women.
Conflict of interest statement
All other authors declared no competing of interest.
Figures


Similar articles
-
Hormonal therapies for early breast cancer: systematic review and economic evaluation.Health Technol Assess. 2007 Jul;11(26):iii-iv, ix-xi, 1-134. doi: 10.3310/hta11260. Health Technol Assess. 2007. PMID: 17610808
-
Aromatase inhibitors for treatment of advanced breast cancer in postmenopausal women.Cochrane Database Syst Rev. 2007 Jan 24;(1):CD003370. doi: 10.1002/14651858.CD003370.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2009 Oct 07;(4):CD003370. doi: 10.1002/14651858.CD003370.pub3. PMID: 17253488 Updated.
-
Aromatase inhibitors in adjuvant therapy for hormone receptor positive breast cancer: a systematic review.Cancer Treat Rev. 2008 Apr;34(2):157-74. doi: 10.1016/j.ctrv.2007.11.001. Epub 2007 Dec 31. Cancer Treat Rev. 2008. PMID: 18164821
-
Systematic review of lapatinib in combination with letrozole compared with other first-line treatments for hormone receptor positive(HR+) and HER2+ advanced or metastatic breast cancer(MBC).Curr Med Res Opin. 2012 Aug;28(8):1263-79. doi: 10.1185/03007995.2012.707643. Epub 2012 Jul 16. Curr Med Res Opin. 2012. PMID: 22738819
-
Systemic therapies for preventing or treating aromatase inhibitor-induced musculoskeletal symptoms in early breast cancer.Cochrane Database Syst Rev. 2022 Jan 10;1(1):CD013167. doi: 10.1002/14651858.CD013167.pub2. Cochrane Database Syst Rev. 2022. PMID: 35005781 Free PMC article.
Cited by
-
Targeting minimal residual disease: a path to cure?Nat Rev Cancer. 2018 Apr;18(4):255-263. doi: 10.1038/nrc.2017.125. Epub 2018 Jan 29. Nat Rev Cancer. 2018. PMID: 29376520 Free PMC article. Review.
-
Cancer Stem Cells: Current Challenges and Future Perspectives.Methods Mol Biol. 2024;2777:1-18. doi: 10.1007/978-1-0716-3730-2_1. Methods Mol Biol. 2024. PMID: 38478332 Review.
-
Commonly Prescribed and Over-the-Counter Drugs as Secondary Causes of Osteoporosis-Part One.Integr Med (Encinitas). 2021 Apr;20(2):8-15. Integr Med (Encinitas). 2021. PMID: 34377089 Free PMC article.
-
Efficacy and Safety of Initial 5 Years of Adjuvant Endocrine Therapy in Postmenopausal Hormone Receptor-Positive Breast Cancer: A Systematic Review and Network Meta-Analysis.Front Pharmacol. 2022 May 30;13:886954. doi: 10.3389/fphar.2022.886954. eCollection 2022. Front Pharmacol. 2022. PMID: 35721183 Free PMC article.
References
-
- Josefsson ML, Leinster SJ. Aromatase inhibitors versus tamoxifen as adjuvant hormonal therapy for oestrogen sensitive early breast cancer in post-menopausal women: meta-analyses of monotherapy, sequenced therapy and extended therapy. Breast. 2010;19(2):76–83. doi: 10.1016/j.breast.2009.12.010. - DOI - PubMed
-
- Davies C, Godwin J, Gray R, Clarke M, Cutter D, Early Breast Cancer Trialists’ Collaborative G et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

