Mouse Nudt13 is a Mitochondrial Nudix Hydrolase with NAD(P)H Pyrophosphohydrolase Activity
- PMID: 28755312
- PMCID: PMC5626787
- DOI: 10.1007/s10930-017-9734-x
Mouse Nudt13 is a Mitochondrial Nudix Hydrolase with NAD(P)H Pyrophosphohydrolase Activity
Abstract
The mammalian NUDT13 protein possesses a sequence motif characteristic of the NADH pyrophosphohydrolase subfamily of Nudix hydrolases. Due to the persistent insolubility of the recombinant product expressed in Escherichia coli, active mouse Nudt13 was expressed in insect cells from a baculovirus vector as a histidine-tagged recombinant protein. In vitro, it efficiently hydrolysed NADH to NMNH and AMP and NADPH to NMNH and 2',5'-ADP and had a marked preference for the reduced pyridine nucleotides. Much lower activity was obtained with other nucleotide substrates tested. K m and k cat values for NADH were 0.34 mM and 7 s-1 respectively. Expression of Nudt13 as an N-terminal fusion to green fluorescent protein revealed that it was targeted exclusively to mitochondria by the N-terminal targeting peptide, suggesting that Nudt13 may act to regulate the concentration of mitochondrial reduced pyridine nucleotide cofactors and the NAD(P)+/NAD(P)H ratio in this organelle and elsewhere. Future studies of the enzymology of pyridine nucleotide metabolism in relation to energy homeostasis, redox control, free radical production and cellular integrity should consider the possible regulatory role of Nudt13.
Keywords: Mitochondria; NADH; Nucleotide metabolism; Nudix; Pyrophosphatase.
Conflict of interest statement
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Figures
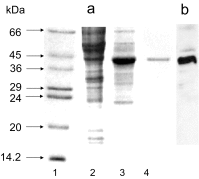
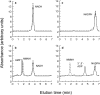

References
-
- Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, Hill DE, Vidal M, Evans JG, Thorburn DR, Carr SA, Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. - DOI - PMC - PubMed
-
- O’Reilly DR, Miller LK, Luckow VA. Baculovirus expression vectors: a laboratory manual. New York: W.H. Freeman; 1992.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

