A first-in-human pharmacodynamic and pharmacokinetic study of a fully human anti-glucagon receptor monoclonal antibody in normal healthy volunteers
- PMID: 28755409
- PMCID: PMC5813272
- DOI: 10.1111/dom.13075
A first-in-human pharmacodynamic and pharmacokinetic study of a fully human anti-glucagon receptor monoclonal antibody in normal healthy volunteers
Abstract
Aims: Glucagon receptor (GCGR) blockers are being investigated as potential therapeutics for type 1 and type 2 diabetes. Here we report the safety, tolerability, pharmacokinetics (PK) and pharmacodynamics (PD) of REGN1193, a fully human glucagon receptor blocking monoclonal antibody from a first-in-human healthy volunteer randomized double-blinded trial.
Methods: Healthy men and women received single ascending doses of REGN1193 ranging from 0.05 to 0.6 mg/kg (n = 42) or placebo (n = 14) intravenously. Safety, tolerability and PK were assessed over 106 days. The glucose-lowering effect of REGN1193 was assessed after induction of hyperglycaemia by serial glucagon challenges.
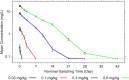
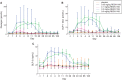
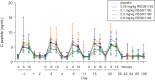
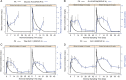
Results: REGN1193 was generally well tolerated. There were small (<3× the upper limit of normal) and transient dose-dependent increases in hepatic aminotransferases. No increase in LDL-C was observed. Hypoglycaemia, assessed as laboratory blood glucose ≤70 mg/dL, occurred in 6/14 (43%) subjects on placebo and 27/42 (57%) on REGN1193 across all dose groups. All episodes of hypoglycaemia were asymptomatic, >50 mg/dL, and did not require treatment or medical assistance. Concentration-time profiles suggest a 2-compartment disposition and marked nonlinearity, consistent with target-mediated clearance. REGN1193 inhibited the glucagon-stimulated glucose increase in a dose-dependent manner. The 0.6 mg/kg dose inhibited the glucagon-induced glucose area under the curve for 0 to 90 minutes (AUC0-90 minutes ) by 80% to 90% on days 3 and 15, while blunting the increase in C-peptide. REGN1193 dose-dependently increased total GLP-1, GLP-2 and glucagon, with plasma levels returning to baseline by day 29 in all dose groups.
Conclusion: REGN1193, a GCGR-blocking monoclonal antibody, produced a safety, tolerability and PK/PD profile suitable for further clinical development. The occurrence of transient elevations in serum hepatic aminotransferases observed here and reported with several small molecule glucagon receptor antagonists suggests an on-target effect of glucagon receptor blockade. The underlying mechanism is unknown.
Keywords: GCGR; REGN1193; glucagon stimulation; phase 1.
© 2017 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
A. Kostic, F. Yang, K. Chan, J. Gromada, and J. B. Harp are employees and shareholders of Regeneron Pharmaceuticals, Inc. George D Yancopoulos is an officer and shareholder of Regeneron Pharmaceuticals, Inc. T.A. King received research funding from Regeneron Pharmaceuticals for clinical trial conduct and advisory board membership, paid to Covance, of which he is an employee.
Figures

References
-
- Gromada J, Franklin I, Wollheim CB. Alpha‐cells of the endocrine pancreas: 35 years of research but the enigma remains. Endocr Rev. 2007;28(1):84–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

