Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice
- PMID: 28755521
- PMCID: PMC5610159
- DOI: 10.1111/bph.13965
Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice
Abstract
Background and purpose: Gut microbiota is essential for the development of the gastrointestinal system, including the enteric nervous system (ENS). Perturbations of gut microbiota in early life have the potential to alter neurodevelopment leading to functional bowel disorders later in life. We examined the hypothesis that gut dysbiosis impairs the structural and functional integrity of the ENS, leading to gut dysmotility in juvenile mice.
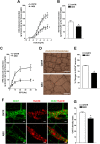
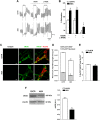
Experimental approach: To induce gut dysbiosis, broad-spectrum antibiotics were administered by gavage to juvenile (3weeks old) male C57Bl/6 mice for 14 days. Bile acid composition in the intestinal lumen was analysed by liquid chromatography-mass spectrometry. Changes in intestinal motility were evaluated by stool frequency, transit of a fluorescent-labelled marker and isometric muscle responses of ileal full-thickness preparations to receptor and non-receptor-mediated stimuli. Alterations in ENS integrity were assessed by immunohistochemistry and Western blot analysis.
Key results: Antibiotic treatment altered gastrointestinal transit, luminal bile acid metabolism and bowel architecture. Gut dysbiosis resulted in distorted glial network, loss of myenteric plexus neurons, altered cholinergic, tachykininergic and nitrergic neurotransmission associated with reduced number of nNOS neurons and different ileal distribution of the toll-like receptor TLR2. Functional defects were partly reversed by activation of TLR2 signalling.
Conclusions and implications: Gut dysbiosis caused complex morpho-functional neuromuscular rearrangements, characterized by structural defects of the ENS and increased tachykininergic neurotransmission. Altogether, our findings support the beneficial role of enteric microbiota for ENS homeostasis instrumental in ensuring proper gut neuromuscular function during critical stages of development.
© 2017 The British Pharmacological Society.
Figures





Similar articles
-
Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice.Gastroenterology. 2020 Jul;159(1):200-213.e8. doi: 10.1053/j.gastro.2020.03.050. Epub 2020 Mar 29. Gastroenterology. 2020. PMID: 32234538 Free PMC article.
-
Toll-like receptor 4 deficiency ameliorates experimental ileitis and enteric neuropathy: Involvement of nitrergic and 5-hydroxytryptaminergic neurotransmission.Br J Pharmacol. 2025 Apr;182(8):1803-1822. doi: 10.1111/bph.17439. Epub 2025 Jan 22. Br J Pharmacol. 2025. PMID: 39842456
-
Sex-dependent alteration of the enteric neuromuscular function after antibiotic-induced dysbiosis in juvenile mice and effect of Lactocaseibacillus rhamnosus GG.Biomed Pharmacother. 2025 Aug;189:118263. doi: 10.1016/j.biopha.2025.118263. Epub 2025 Jun 13. Biomed Pharmacother. 2025. PMID: 40516333
-
Gastrointestinal neuromuscular apparatus: An underestimated target of gut microbiota.World J Gastroenterol. 2016 Dec 7;22(45):9871-9879. doi: 10.3748/wjg.v22.i45.9871. World J Gastroenterol. 2016. PMID: 28018095 Free PMC article. Review.
-
Altered microbiome influence on the enteric neuromuscular system in amyotrophic lateral sclerosis (ALS).Int Rev Neurobiol. 2025;180:95-123. doi: 10.1016/bs.irn.2025.04.006. Epub 2025 May 16. Int Rev Neurobiol. 2025. PMID: 40414644 Review.
Cited by
-
Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis.Int J Mol Sci. 2019 Mar 25;20(6):1482. doi: 10.3390/ijms20061482. Int J Mol Sci. 2019. PMID: 30934533 Free PMC article. Review.
-
Involvement of Enteric Glia in Small Intestine Neuromuscular Dysfunction of Toll-Like Receptor 4-Deficient Mice.Cells. 2020 Mar 31;9(4):838. doi: 10.3390/cells9040838. Cells. 2020. PMID: 32244316 Free PMC article.
-
Innovative Animal Model of DSS-Induced Ulcerative Colitis in Pseudo Germ-Free Mice.Cells. 2020 Dec 1;9(12):2571. doi: 10.3390/cells9122571. Cells. 2020. PMID: 33271873 Free PMC article.
-
Probiotics for functional constipation in children: an overview of overlapping systematic reviews.Front Cell Infect Microbiol. 2024 Jan 8;13:1323521. doi: 10.3389/fcimb.2023.1323521. eCollection 2023. Front Cell Infect Microbiol. 2024. PMID: 38259973 Free PMC article. Review.
-
Selenium Mitigates Ammonia-Induced Neurotoxicity by Suppressing Apoptosis, Immune Imbalance, and Gut Microbiota-Driven Metabolic Disturbance in Fattening Pigs.Biol Trace Elem Res. 2023 Jul;201(7):3341-3355. doi: 10.1007/s12011-022-03434-w. Epub 2022 Oct 13. Biol Trace Elem Res. 2023. PMID: 36224318 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

