5- or/and 20-O-alkyl-2,3-dehydrosilybins: Synthesis and biological profiles on prostate cancer cell models
- PMID: 28756013
- PMCID: PMC5568090
- DOI: 10.1016/j.bmc.2017.07.035
5- or/and 20-O-alkyl-2,3-dehydrosilybins: Synthesis and biological profiles on prostate cancer cell models
Abstract
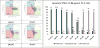
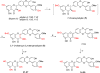
To investigate the effects of alkylation at 5-OH and 20-OH of 2,3-dehydrosilybin on prostate cancer cell proliferation, the synthetic approaches to 5- or/and 20-O-alkyl-2,3-dehydrosilybins, through a multi-step sequence from commercially available silybin, have been successfully developed. The first three reactions in the syntheses were completed through a one-pot procedure by managing anaerobic and aerobic conditions. With these synthetic methods in hand, twenty-one 2,3-dehydrosilybins, including seven 20-O-alkyl, seven 5,20-O-dialkyl, and seven 5-O-alkyl-2,3-dehydrosilybins, have been achieved for the evaluation of their biological profiles. Our WST-1 cell proliferation assay data indicate that nineteen out of the twenty-one 2,3-dehydrosilybins possess significantly improved antiproliferative potency as compared with silybin toward both androgen-sensitive (LNCaP) and androgen-insensitive prostate cancer cell lines (PC-3 and DU145). 5-O-Alkyl-2,3-dehydrosilybins were identified as the optimal subgroup that can consistently inhibit cell proliferation in three prostate cancer cell models with all IC50 values lower than 8µM. Our flow cytometry-based assays also demonstrate that 5-O-heptyl-2,3-dehydrosilybin effectively arrests the cell cycle in the G0/G1 phase and activates PC-3 cell apoptosis.
Keywords: 2,3-Dehydrosilybin derivatives; Anti-proliferative activity; Cell apoptosis; Cell cycle regulation; Prostate cancer; Synthesis.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures


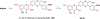
Similar articles
-
O-Aminoalkyl-O-Trimethyl-2,3-Dehydrosilybins: Synthesis and In Vitro Effects Towards Prostate Cancer Cells.Molecules. 2018 Nov 29;23(12):3142. doi: 10.3390/molecules23123142. Molecules. 2018. PMID: 30501133 Free PMC article.
-
3-O-Alkyl-2,3-dehydrosilibinins: Two synthetic approaches and in vitro effects toward prostate cancer cells.Bioorg Med Chem Lett. 2016 Jul 15;26(14):3226-3231. doi: 10.1016/j.bmcl.2016.05.069. Epub 2016 May 24. Bioorg Med Chem Lett. 2016. PMID: 27261177 Free PMC article.
-
Silibinin derivatives as anti-prostate cancer agents: Synthesis and cell-based evaluations.Eur J Med Chem. 2016 Feb 15;109:36-46. doi: 10.1016/j.ejmech.2015.12.041. Epub 2015 Dec 24. Eur J Med Chem. 2016. PMID: 26748997 Free PMC article.
-
A new class of flavonol-based anti-prostate cancer agents: Design, synthesis, and evaluation in cell models.Bioorg Med Chem Lett. 2016 Sep 1;26(17):4241-5. doi: 10.1016/j.bmcl.2016.07.050. Epub 2016 Jul 22. Bioorg Med Chem Lett. 2016. PMID: 27476422 Free PMC article.
-
3-O-Substituted-3',4',5'-trimethoxyflavonols: Synthesis and cell-based evaluation as anti-prostate cancer agents.Bioorg Med Chem. 2017 Sep 1;25(17):4768-4777. doi: 10.1016/j.bmc.2017.07.022. Epub 2017 Jul 21. Bioorg Med Chem. 2017. PMID: 28760528 Free PMC article.
Cited by
-
Elevated phospholipase D activity in androgen-insensitive prostate cancer cells promotes both survival and metastatic phenotypes.Cancer Lett. 2018 Jun 1;423:28-35. doi: 10.1016/j.canlet.2018.03.006. Epub 2018 Mar 8. Cancer Lett. 2018. PMID: 29524555 Free PMC article.
-
7-O-tyrosyl Silybin Derivatives as a Novel Set of Anti-Prostate Cancer Compounds.Antioxidants (Basel). 2024 Mar 29;13(4):418. doi: 10.3390/antiox13040418. Antioxidants (Basel). 2024. PMID: 38671866 Free PMC article.
-
Nitrogen-containing derivatives of O-tetramethylquercetin: Synthesis and biological profiles in prostate cancer cell models.Bioorg Chem. 2019 Jun;87:227-239. doi: 10.1016/j.bioorg.2019.03.047. Epub 2019 Mar 19. Bioorg Chem. 2019. PMID: 30904813 Free PMC article.
-
Design, Synthesis and Biological Evaluation of Glycosylated Derivatives of Silibinin as Potential Anti-Tumor Agents.Drug Des Devel Ther. 2023 Jul 11;17:2063-2076. doi: 10.2147/DDDT.S404036. eCollection 2023. Drug Des Devel Ther. 2023. PMID: 37457888 Free PMC article.
-
O-Aminoalkyl-O-Trimethyl-2,3-Dehydrosilybins: Synthesis and In Vitro Effects Towards Prostate Cancer Cells.Molecules. 2018 Nov 29;23(12):3142. doi: 10.3390/molecules23123142. Molecules. 2018. PMID: 30501133 Free PMC article.
References
-
- Pelter A, Haensel R. Tetrahedron Lett. 1968;2911
-
- Vue B, Chen Q-H. Cur Med Chem. 2016;23:3925. - PubMed
-
- Kim N-C, Graf TN, Sparacino CM, Wani MC, Wall ME. Org Biomol Chem. 2003;1:1684. - PubMed
-
- Flora K, Hahn M, Rosen H, Benner K. Am J Gastroenterol. 1998;93:139. - PubMed
-
- Abenavoli L, Capasso R, Milic N, Capasso F. Phytother Res. 2010;24:1423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

