Reduction of podocyte globotriaosylceramide content in adult male patients with Fabry disease with amenable GLA mutations following 6 months of migalastat treatment
- PMID: 28756410
- PMCID: PMC5740534
- DOI: 10.1136/jmedgenet-2017-104826
Reduction of podocyte globotriaosylceramide content in adult male patients with Fabry disease with amenable GLA mutations following 6 months of migalastat treatment
Abstract
Objective: Deficiency of α-galactosidase A (αGal-A) in Fabry disease leads to the accumulation mainly of globotriaosylceramide (GL3) in multiple renal cell types. Glomerular podocytes are relatively resistant to clearance of GL3 inclusions by enzyme replacement therapy (ERT). Migalastat, an orally bioavailable small molecule capable of chaperoning misfolded αGal-A to lysosomes, is approved in the European Union for the long-term treatment of patients with Fabry disease and amenable GLA (α-galactosidase A enzyme) mutations. We aimed to examine if migalastat reduces GL3 content of podocytes in Fabry disease.
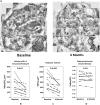
Methods and analysis: We compared paired renal biopsies of eight adult men with amenable Fabry disease mutations at baseline and after 6 months of treatment with 150 mg migalastat every other day using quantitative unbiased electron microscopic morphometric methods.
Results: Migalastat treatment led to a reduction in mean total GL3 inclusion volume per podocyte in renal biopsies from baseline to 6 months. This reduction correlated precisely with reduced mean podocyte volume. There was also a direct relationship between reduction in podocyte foot process width and the reduction in mean total podocyte GL3 content following 6 months of migalastat treatment, suggestive of reduced podocyte injury.
Conclusion: Migalastat treatment of 6 months duration in eight male patients with Fabry disease demonstrated effective GL3 clearance from the podocyte, an important and relatively ERT-resistant glomerular cell.
Keywords: Fabry; GL3; chaperone; globotriaosylceramide; migalastat; podocyte.
© Article author(s) (or their employer(s) unless otherwise stated in the text of the article) 2017. All rights reserved. No commercial use is permitted unless otherwise expressly granted.
Conflict of interest statement
Competing interests: BN is a recipient of investigator initiated Genzyme research grants, a consultant to Genzyme and has received speaker’s honoraria and travel support from Genzyme. He is also a member of the Medical Advisory Board of Amicus and performs kidney biopsy studies for Amicus. These interests have been reviewed and managed by the University of Washington in accordance to its conflict of interest policies. MM is a member of the Genzyme sponsored North American Fabry Registry Advisory Board, a recipient of investigator initiated Genzyme research grants, a consultant to Genzyme for clinical trial design, and a speaker at Genzyme educational meetings. These interests have been reviewed and managed by the University of Minnesota in accordance to its conflict of interest policies. He is also a consultant to and performs kidney biopsy studies for Amicus and has served as grant reviewer for Shire.
Figures



Similar articles
-
Chaperone Therapy in Fabry Disease.Int J Mol Sci. 2022 Feb 8;23(3):1887. doi: 10.3390/ijms23031887. Int J Mol Sci. 2022. PMID: 35163813 Free PMC article. Review.
-
One Year of Enzyme Replacement Therapy Reduces Globotriaosylceramide Inclusions in Podocytes in Male Adult Patients with Fabry Disease.PLoS One. 2016 Apr 15;11(4):e0152812. doi: 10.1371/journal.pone.0152812. eCollection 2016. PLoS One. 2016. PMID: 27081853 Free PMC article. Clinical Trial.
-
Migalastat: A Review in Fabry Disease.Drugs. 2019 Apr;79(5):543-554. doi: 10.1007/s40265-019-01090-4. Drugs. 2019. PMID: 30875019 Free PMC article. Review.
-
Accumulation of Globotriaosylceramide in Podocytes in Fabry Nephropathy Is Associated with Progressive Podocyte Loss.J Am Soc Nephrol. 2020 Apr;31(4):865-875. doi: 10.1681/ASN.2019050497. Epub 2020 Mar 3. J Am Soc Nephrol. 2020. PMID: 32127409 Free PMC article.
-
Oral pharmacological chaperone migalastat compared with enzyme replacement therapy in Fabry disease: 18-month results from the randomised phase III ATTRACT study.J Med Genet. 2017 Apr;54(4):288-296. doi: 10.1136/jmedgenet-2016-104178. Epub 2016 Nov 10. J Med Genet. 2017. PMID: 27834756 Free PMC article. Clinical Trial.
Cited by
-
Biomarkers for Monitoring Renal Damage Due to Fabry Disease in Patients Treated with Migalastat: A Review for Nephrologists.Genes (Basel). 2022 Sep 28;13(10):1751. doi: 10.3390/genes13101751. Genes (Basel). 2022. PMID: 36292636 Free PMC article. Review.
-
A review and recommendations for oral chaperone therapy in adult patients with Fabry disease.Orphanet J Rare Dis. 2024 Jan 18;19(1):16. doi: 10.1186/s13023-024-03028-w. Orphanet J Rare Dis. 2024. PMID: 38238782 Free PMC article. Review.
-
A novel unbiased method reveals progressive podocyte globotriaosylceramide accumulation and loss with age in females with Fabry disease.Kidney Int. 2022 Jul;102(1):173-182. doi: 10.1016/j.kint.2022.03.023. Epub 2022 Apr 26. Kidney Int. 2022. PMID: 35483528 Free PMC article.
-
Fabry disease - a multisystemic disease with gastrointestinal manifestations.Gut Microbes. 2022 Jan-Dec;14(1):2027852. doi: 10.1080/19490976.2022.2027852. Gut Microbes. 2022. PMID: 35090382 Free PMC article. Review.
-
Chaperone Therapy in Fabry Disease.Int J Mol Sci. 2022 Feb 8;23(3):1887. doi: 10.3390/ijms23031887. Int J Mol Sci. 2022. PMID: 35163813 Free PMC article. Review.
References
-
- Desnick RJ, Allen KY, Desnick SJ, Raman MK, Bernlohr RW, Krivit W. Fabry’s disease: enzymatic diagnosis of hemizygotes and heterozygotes. Alpha-galactosidase activities in plasma, serum, urine, and leukocytes. J Lab Clin Med 1973;81:157–71. - PubMed
-
- Germain DP, Waldek S, Banikazemi M, Bushinsky DA, Charrow J, Desnick RJ, Lee P, Loew T, Vedder AC, Abichandani R, Wilcox WR, Guffon N. Sustained, long-term renal stabilization after 54 months of agalsidase beta therapy in patients with Fabry disease. J Am Soc Nephrol 2007;18:1547–57. 10.1681/ASN.2006080816 - DOI - PubMed
-
- Banikazemi M, Bultas J, Waldek S, Wilcox WR, Whitley CB, McDonald M, Finkel R, Packman S, Bichet DG, Warnock DG, Desnick RJ. Fabry Disease Clinical Trial Study Group. Agalsidase-beta therapy for advanced Fabry disease: a randomized trial. Ann Intern Med 2007;146:77–86. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
