Pathogen-reduced platelets for the prevention of bleeding
- PMID: 28756627
- PMCID: PMC5558872
- DOI: 10.1002/14651858.CD009072.pub3
Pathogen-reduced platelets for the prevention of bleeding
Abstract
Background: Platelet transfusions are used to prevent and treat bleeding in people who are thrombocytopenic. Despite improvements in donor screening and laboratory testing, a small risk of viral, bacterial, or protozoal contamination of platelets remains. There is also an ongoing risk from newly emerging blood transfusion-transmitted infections for which laboratory tests may not be available at the time of initial outbreak.One solution to reduce the risk of blood transfusion-transmitted infections from platelet transfusion is photochemical pathogen reduction, in which pathogens are either inactivated or significantly depleted in number, thereby reducing the chance of transmission. This process might offer additional benefits, including platelet shelf-life extension, and negate the requirement for gamma-irradiation of platelets. Although current pathogen-reduction technologies have been proven to reduce pathogen load in platelet concentrates, a number of published clinical studies have raised concerns about the effectiveness of pathogen-reduced platelets for post-transfusion platelet count recovery and the prevention of bleeding when compared with standard platelets.This is an update of a Cochrane review first published in 2013.
Objectives: To assess the effectiveness of pathogen-reduced platelets for the prevention of bleeding in people of any age requiring platelet transfusions.
Search methods: We searched for randomised controlled trials (RCTs) in the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 9), MEDLINE (from 1946), Embase (from 1974), CINAHL (from 1937), the Transfusion Evidence Library (from 1950), and ongoing trial databases to 24 October 2016.
Selection criteria: We included RCTs comparing the transfusion of pathogen-reduced platelets with standard platelets, or comparing different types of pathogen-reduced platelets.
Data collection and analysis: We used the standard methodological procedures expected by Cochrane.
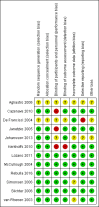
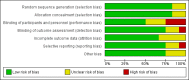
Main results: We identified five new trials in this update of the review. A total of 15 trials were eligible for inclusion in this review, 12 completed trials (2075 participants) and three ongoing trials. Ten of the 12 completed trials were included in the original review. We did not identify any RCTs comparing the transfusion of one type of pathogen-reduced platelets with another.Nine trials compared Intercept® pathogen-reduced platelets to standard platelets, two trials compared Mirasol® pathogen-reduced platelets to standard platelets; and one trial compared both pathogen-reduced platelets types to standard platelets. Three RCTs were randomised cross-over trials, and nine were parallel-group trials. Of the 2075 participants enrolled in the trials, 1981 participants received at least one platelet transfusion (1662 participants in Intercept® platelet trials and 319 in Mirasol® platelet trials).One trial included children requiring cardiac surgery (16 participants) or adults requiring a liver transplant (28 participants). All of the other participants were thrombocytopenic individuals who had a haematological or oncological diagnosis. Eight trials included only adults.Four of the included studies were at low risk of bias in every domain, while the remaining eight included studies had some threats to validity.Overall, the quality of the evidence was low to high across different outcomes according to GRADE methodology.We are very uncertain as to whether pathogen-reduced platelets increase the risk of any bleeding (World Health Organization (WHO) Grade 1 to 4) (5 trials, 1085 participants; fixed-effect risk ratio (RR) 1.09, 95% confidence interval (CI) 1.02 to 1.15; I2 = 59%, random-effect RR 1.14, 95% CI 0.93 to 1.38; I2 = 59%; low-quality evidence).There was no evidence of a difference between pathogen-reduced platelets and standard platelets in the incidence of clinically significant bleeding complications (WHO Grade 2 or higher) (5 trials, 1392 participants; RR 1.10, 95% CI 0.97 to 1.25; I2 = 0%; moderate-quality evidence), and there is probably no difference in the risk of developing severe bleeding (WHO Grade 3 or higher) (6 trials, 1495 participants; RR 1.24, 95% CI 0.76 to 2.02; I2 = 32%; moderate-quality evidence).There is probably no difference between pathogen-reduced platelets and standard platelets in the incidence of all-cause mortality at 4 to 12 weeks (6 trials, 1509 participants; RR 0.81, 95% CI 0.50 to 1.29; I2 = 26%; moderate-quality evidence).There is probably no difference between pathogen-reduced platelets and standard platelets in the incidence of serious adverse events (7 trials, 1340 participants; RR 1.09, 95% CI 0.88 to 1.35; I2 = 0%; moderate-quality evidence). However, no bacterial transfusion-transmitted infections occurred in the six trials that reported this outcome.Participants who received pathogen-reduced platelet transfusions had an increased risk of developing platelet refractoriness (7 trials, 1525 participants; RR 2.94, 95% CI 2.08 to 4.16; I2 = 0%; high-quality evidence), though the definition of platelet refractoriness differed between trials.Participants who received pathogen-reduced platelet transfusions required more platelet transfusions (6 trials, 1509 participants; mean difference (MD) 1.23, 95% CI 0.86 to 1.61; I2 = 27%; high-quality evidence), and there was probably a shorter time interval between transfusions (6 trials, 1489 participants; MD -0.42, 95% CI -0.53 to -0.32; I2 = 29%; moderate-quality evidence). Participants who received pathogen-reduced platelet transfusions had a lower 24-hour corrected-count increment (7 trials, 1681 participants; MD -3.02, 95% CI -3.57 to -2.48; I2 = 15%; high-quality evidence).None of the studies reported quality of life.We did not evaluate any economic outcomes.There was evidence of subgroup differences in multiple transfusion trials between the two pathogen-reduced platelet technologies assessed in this review (Intercept® and Mirasol®) for all-cause mortality and the interval between platelet transfusions (favouring Intercept®).
Authors' conclusions: Findings from this review were based on 12 trials, and of the 1981 participants who received a platelet transfusion only 44 did not have a haematological or oncological diagnosis.In people with haematological or oncological disorders who are thrombocytopenic due to their disease or its treatment, we found high-quality evidence that pathogen-reduced platelet transfusions increase the risk of platelet refractoriness and the platelet transfusion requirement. We found moderate-quality evidence that pathogen-reduced platelet transfusions do not affect all-cause mortality, the risk of clinically significant or severe bleeding, or the risk of a serious adverse event. There was insufficient evidence for people with other diagnoses.All three ongoing trials are in adults (planned recruitment 1375 participants) with a haematological or oncological diagnosis.
Conflict of interest statement
Lise Estcourt is partly funded by NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Reem Malouf is partly funded by NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Sally Hopewell is partly funded by NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Marialena Trivella is partly funded by NIHR Cochrane Programme Grant ‐ Safe and Appropriate Use of Blood Components.
Carolyn Doree: None known.
Simon Stanworth: None known.
Michael Murphy: None known.
Figures


























Update of
-
Pathogen-reduced platelets for the prevention of bleeding.Cochrane Database Syst Rev. 2013 Mar 28;(3):CD009072. doi: 10.1002/14651858.CD009072.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2017 Jul 30;7:CD009072. doi: 10.1002/14651858.CD009072.pub3. PMID: 23543569 Updated.
References
References to studies included in this review
-
- Agliastro RE, Francisci G, Bonaccorso R, Spicola D, Ziino O, Arico M, et al. Clinical study in pediatric hemato‐oncology patients: efficacy of pathogen inactivated buffy coat platelets versus aphaeresis platelets. Transfusion 2006;46(9s):117A. Abstract No. SP246.
-
- Ambruso DR, Thurman G, Marschner S, Goodrich RP. Lack of antibody formation to platelet neoantigens after transfusion of riboflavin and ultraviolet light‐treated platelet concentrates. Transfusion 2009;49(12):2631‐6. [PUBMED: 19694996] - PubMed
- Goodrich R, Roberts T, Follea G. Clinical evaluation of Mirasol PRT treated apheresis platelets in thrombocytopenic patients. Transfusion 2008;48(Suppl):20A. Abstract No. S49‐020G.
- Mirasol Clinical Evaluation Study Group. A randomized controlled clinical trial evaluating the performance and safety of platelets treated with MIRASOL pathogen reduction technology. Transfusion 2010;50(11):2362‐75. [NCT00263809; PUBMED: 20492615] - PubMed
- NCT00263809. Safety and performance of MIRASOL® PRT treated platelet transfusion products. clinicaltrials.gov/ct2/show/NCT00263809 first received 7 December 7 2005.
-
- Francisci G, Bonaccorso R, Bellavia D, D'Alia G, Fiandaca T, Giancana A, et al. Clinical trial on the use of pathogen inactivated platelets, with Helinx® technology, in cardio paediatric surgery and cirrhotic patients. Transfusion 2004;44(s1):17A. Abstract No. S48‐0301.
-
- Janetzko K, Cazenave JP, Kluter H, Kientz D, Michel M, Beris P, et al. Therapeutic efficacy and safety of photochemically treated apheresis platelets processed with an optimized integrated set. Transfusion 2005;45(9):1443‐52. [PUBMED: 16131376] - PubMed
-
- Johansson PI, Simonsen A, Ostrowski SR, Deberdt L, Marschner S, Goodrich R. TEG as a surrogate marker for the haemostatic function of PRT treated platelets in thrombocytopenic patients. Transfusion 2012;52(Suppl 3):67A. Abstract No. SP34.
- Johansson PI, Simonsen AC, Brown PN, Ostrowski SR, Deberdt L, Hoydonck P, et al. A pilot study to assess the hemostatic function of pathogen‐reduced platelets in patients with thrombocytopenia. Transfusion 13;53(9):2043‐52. - PubMed
- NCT01368211. Mirasol‐treated platelets ‐ (Pathogen Reduction Extended Storage Study) (PRESS). clinicaltrials.gov/ct2/show/NCT01368211 first received 6 June 2011.
References to studies excluded from this review
-
- Andreu G, Norol F, Schooneman F, Coffe C, Pouthier F, Soulier J, et al. Prevention of HLA alloimmunization using UV‐B irradiated platelet concentrates (PC): results of a prospective randomized clinical trial. Transfusion 1993;33(9s):73S. Abstract No. S283.
-
- AuBuchon JP, Herschel L, Roger J, Taylor H, Whitley P, Li J, et al. Efficacy of apheresis platelets treated with riboflavin and ultraviolet light for pathogen reduction. Transfusion 2004;44(s1):16A‐7A. Abstract No. S47‐0301. - PubMed
- AuBuchon JP, Herschel L, Roger J, Taylor H, Whitley P, Li J, et al. Efficacy of apheresis platelets treated with riboflavin and ultraviolet light for pathogen reduction. Transfusion 2005;45(8):1335‐41. [PUBMED: 16078923] - PubMed
-
- Ayupova F. Clinical application of transfused platelet concentrates treated with Intercepttm blood system technology‐experience from ufa center. 33rd International Congress of the International Society of Blood Transfusion, in Conjunction with the 33rd Congress of the KSBT and the 2014 Congress of the Korean Hematology Societies Seoul, South Korea 2014;107:130‐1.
-
- Berger K, Bauer M, Schopohl D, Henschler R, Ostermann H. Model calculations to quantify clinical and economic effects of pathogen inactivation in platelet concentrates. Onkologie 2013;39(1‐2):53‐9. - PubMed
-
- Blundell EL, Pamphilon DH, Fraser ID, Menitove JE, Greenwalt TJ, Snyder EL, et al. A prospective, randomized study of the use of platelet concentrates irradiated with ultraviolet‐B light in patients with hematologic malignancy. Transfusion 1996;36(4):296‐302. [PUBMED: 8623127] - PubMed
References to ongoing studies
-
- Phase III, randomised, double‐blind, multi‐centre clinical trial on clinical efficacy and safety of platelet concentrates treated with the THERAFLEX UV Platelets procedure in comparison to conventional platelet components (Capture). Ongoing studyJune 2016.
-
- NCT02783313. Pathogen Reduction Evaluation & Predictive Analytical Rating Score (PREPAReS). www.clinicaltrials.gov/ct2/show/NCT02783313 first received 11 April 11 2016. [NTR2106]
- NTR2106. The PREPAReS Study: Pathogen Reduction Evaluation & Predictive Analytical Rating Score. www.trialregister.nl/trialreg/admin/rctview.asp?TC=2106 first received 13 November 2009.
- Ypma PF, Meer PF, Heddle NM, Hilten JA, Stijnen T, Middleburg RA, et al. A study protocol for a randomised controlled trial evaluating clinical effects of platelet transfusion products: the Pathogen Reduction Evaluation and Predictive Analytical Rating Score (PREPAReS) trial. BMJ Open 2016;6:e010156. - PMC - PubMed
-
- NCT01789762. Evaluation of the efficacy of platelets treated with pathogen inactivation process (EFFIPAP). www.clinicaltrials.gov/ct2/show/NCT01789762 first received 8 February 2013.
Additional references
-
- Benjamin RJ, Dy B, Perez J, Eder AF, Wagne SJ. Bacterial culture of apheresis platelets: a mathematical model of the residual rate of contamination based on unconfirmed positive results. International Society of Blood Transfusion 2014;106:23‐30. - PubMed
-
- Blajchman MA, Vamvakas EC. The continuing risk of transfusion‐transmitted infections. New England Journal of Medicine 2006;355:1303‐5. - PubMed
-
- Bolton‐Maggs P (Ed), Poles D, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2014 Annual SHOT Report. SHOT, 2015.
-
- Bolton‐Maggs P (Ed), Poles P, et al. on behalf of the Serious Hazards of Transfusion (SHOT) Steering Group. The 2015 Annual SHOT Report. SHOT, 2016.
-
- Cazenave JP, Isola H, Waller C, Mendel I, Kientz D, Laforêt M, et al. Use of additive solutions and pathogen inactivation treatment of platelet components in a regional blood center: impact on patient outcomes and component utilization during a 3‐year period. Transfusion 2011;51:622‐9. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

