Retinoic acid improves nephrotoxic serum-induced glomerulonephritis through activation of podocyte retinoic acid receptor α
- PMID: 28756872
- PMCID: PMC5696080
- DOI: 10.1016/j.kint.2017.04.026
Retinoic acid improves nephrotoxic serum-induced glomerulonephritis through activation of podocyte retinoic acid receptor α
Abstract
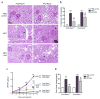
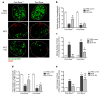
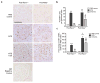
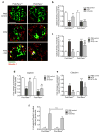
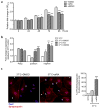
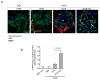
Proliferation of glomerular epithelial cells, including podocytes, is a key histologic feature of crescentic glomerulonephritis. We previously found that retinoic acid (RA) inhibits proliferation and induces differentiation of podocytes by activating RA receptor-α (RARα) in a murine model of HIV-associated nephropathy. Here, we examined whether RA would similarly protect podocytes against nephrotoxic serum-induced crescentic glomerulonephritis and whether this effect was mediated by podocyte RARα. RA treatment markedly improved renal function and reduced the number of crescentic lesions in nephritic wild-type mice, while this protection was largely lost in mice with podocyte-specific ablation of Rara (Pod-Rara knockout). At a cellular level, RA significantly restored the expression of podocyte differentiation markers in nephritic wild-type mice, but not in nephritic Pod-Rara knockout mice. Furthermore, RA suppressed the expression of cell injury, proliferation, and parietal epithelial cell markers in nephritic wild-type mice, all of which were significantly dampened in nephritic Pod-Rara knockout mice. Interestingly, RA treatment led to the coexpression of podocyte and parietal epithelial cell markers in a small subset of glomerular cells in nephritic mice, suggesting that RA may induce transdifferentiation of parietal epithelial cells toward a podocyte phenotype. In vitro, RA directly inhibited the proliferation of parietal epithelial cells and enhanced the expression of podocyte markers. In vivo lineage tracing of labeled parietal epithelial cells confirmed that RA increased the number of parietal epithelial cells expressing podocyte markers in nephritic glomeruli. Thus, RA attenuates crescentic glomerulonephritis primarily through RARα-mediated protection of podocytes and in part through the inhibition of parietal epithelial cell proliferation and induction of their transdifferentiation into podocytes.
Keywords: crescentic glomerulonephritis; parietal epithelial cells; podocytes; proliferation; retinoic acid receptor-alpha; transdifferentiation.
Published by Elsevier Inc.
Conflict of interest statement
Figures


Similar articles
-
Disparate roles of retinoid acid signaling molecules in kidney disease.Am J Physiol Renal Physiol. 2021 May 1;320(5):F683-F692. doi: 10.1152/ajprenal.00045.2021. Epub 2021 Mar 1. Am J Physiol Renal Physiol. 2021. PMID: 33645319 Free PMC article. Review.
-
Podocyte-specific deletion of signal transducer and activator of transcription 3 attenuates nephrotoxic serum-induced glomerulonephritis.Kidney Int. 2013 Nov;84(5):950-61. doi: 10.1038/ki.2013.197. Epub 2013 Jul 10. Kidney Int. 2013. PMID: 23842188 Free PMC article.
-
Retinoic acid inhibits HIV-1-induced podocyte proliferation through the cAMP pathway.J Am Soc Nephrol. 2007 Jan;18(1):93-102. doi: 10.1681/ASN.2006070727. Epub 2006 Dec 20. J Am Soc Nephrol. 2007. PMID: 17182884 Free PMC article.
-
Role of the retinoic acid receptor-α in HIV-associated nephropathy.Kidney Int. 2011 Mar;79(6):624-634. doi: 10.1038/ki.2010.470. Epub 2010 Dec 8. Kidney Int. 2011. PMID: 21150871 Free PMC article.
-
Novel therapeutic perspectives for crescentic glomerulonephritis through targeting parietal epithelial cell activation and proliferation.Expert Opin Ther Targets. 2023 Jan;27(1):55-69. doi: 10.1080/14728222.2023.2177534. Epub 2023 Feb 16. Expert Opin Ther Targets. 2023. PMID: 36738160 Review.
Cited by
-
Podocyte-specific knockout of the neonatal Fc receptor (FcRn) results in differential protection depending on the model of glomerulonephritis.PLoS One. 2020 Dec 28;15(12):e0230401. doi: 10.1371/journal.pone.0230401. eCollection 2020. PLoS One. 2020. PMID: 33370294 Free PMC article.
-
Vitamin A and retinoid signaling in the kidneys.Pharmacol Ther. 2023 Aug;248:108481. doi: 10.1016/j.pharmthera.2023.108481. Epub 2023 Jun 17. Pharmacol Ther. 2023. PMID: 37331524 Free PMC article. Review.
-
Genome-wide meta-analysis identifies new candidate genes for sickle cell disease nephropathy.Blood Adv. 2023 Sep 12;7(17):4782-4793. doi: 10.1182/bloodadvances.2022007451. Blood Adv. 2023. PMID: 36399516 Free PMC article.
-
Glucocorticoids Inhibit EGFR Signaling Activation in Podocytes in Anti-GBM Crescentic Glomerulonephritis.Front Med (Lausanne). 2022 Feb 10;9:697443. doi: 10.3389/fmed.2022.697443. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35223886 Free PMC article.
-
Disparate roles of retinoid acid signaling molecules in kidney disease.Am J Physiol Renal Physiol. 2021 May 1;320(5):F683-F692. doi: 10.1152/ajprenal.00045.2021. Epub 2021 Mar 1. Am J Physiol Renal Physiol. 2021. PMID: 33645319 Free PMC article. Review.
References
-
- Le Hir M, Keller C, Eschmann V, et al. Podocyte bridges between the tuft and Bowman’s capsule: an early event in experimental crescentic glomerulonephritis. J Am Soc Nephrol. 2001;12:2060–2071. - PubMed
-
- Moeller MJ, Soofi A, Hartmann I, et al. Podocytes populate cellular crescents in a murine model of inflammatory glomerulonephritis. J Am Soc Nephrol. 2004;15:61–67. - PubMed
-
- Bariety J, Bruneval P, Meyrier A, et al. Podocyte involvement in human immune crescentic glomerulonephritis. Kidney Int. 2005;68:1109–1119. - PubMed
-
- Ding M, Cui S, Li C, et al. Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med. 2006;12:1081–1087. - PubMed
-
- Griffin SV, Krofft RD, Pippin JW, et al. Limitation of podocyte proliferation improves renal function in experimental crescentic glomerulonephritis. Kidney Int. 2005;67:977–986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

