Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240)
- PMID: 28756902
- PMCID: PMC5714293
- DOI: 10.1016/S0140-6736(17)31607-0
Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240)
Abstract
Background: On Aug 14, 2014, the US Food and Drug Administration approved the antiangiogenesis drug bevacizumab for women with advanced cervical cancer on the basis of improved overall survival (OS) after the second interim analysis (in 2012) of 271 deaths in the Gynecologic Oncology Group (GOG) 240 trial. In this study, we report the prespecified final analysis of the primary objectives, OS and adverse events.
Methods: In this randomised, controlled, open-label, phase 3 trial, we recruited patients with metastatic, persistent, or recurrent cervical carcinoma from 81 centres in the USA, Canada, and Spain. Inclusion criteria included a GOG performance status score of 0 or 1; adequate renal, hepatic, and bone marrow function; adequately anticoagulated thromboembolism; a urine protein to creatinine ratio of less than 1; and measurable disease. Patients who had received chemotherapy for recurrence and those with non-healing wounds or active bleeding conditions were ineligible. We randomly allocated patients 1:1:1:1 (blocking used; block size of four) to intravenous chemotherapy of either cisplatin (50 mg/m2 on day 1 or 2) plus paclitaxel (135 mg/m2 or 175 mg/m2 on day 1) or topotecan (0·75 mg/m2 on days 1-3) plus paclitaxel (175 mg/m2 on day 1) with or without intravenous bevacizumab (15 mg/kg on day 1) in 21 day cycles until disease progression, unacceptable toxic effects, voluntary withdrawal by the patient, or complete response. We stratified randomisation by GOG performance status (0 vs 1), previous radiosensitising platinum-based chemotherapy, and disease status (recurrent or persistent vs metastatic). We gave treatment open label. Primary outcomes were OS (analysed in the intention-to-treat population) and adverse events (analysed in all patients who received treatment and submitted adverse event information), assessed at the second interim and final analysis by the masked Data and Safety Monitoring Board. The cutoff for final analysis was 450 patients with 346 deaths. This trial is registered with ClinicalTrials.gov, number NCT00803062.
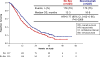
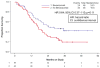
Findings: Between April 6, 2009, and Jan 3, 2012, we enrolled 452 patients (225 [50%] in the two chemotherapy-alone groups and 227 [50%] in the two chemotherapy plus bevacizumab groups). By March 7, 2014, 348 deaths had occurred, meeting the prespecified cutoff for final analysis. The chemotherapy plus bevacizumab groups continued to show significant improvement in OS compared with the chemotherapy-alone groups: 16·8 months in the chemotherapy plus bevacizumab groups versus 13·3 months in the chemotherapy-alone groups (hazard ratio 0·77 [95% CI 0·62-0·95]; p=0·007). Final OS among patients not receiving previous pelvic radiotherapy was 24·5 months versus 16·8 months (0·64 [0·37-1·10]; p=0·11). Postprogression OS was not significantly different between the chemotherapy plus bevacizumab groups (8·4 months) and chemotherapy-alone groups (7·1 months; 0·83 [0·66-1·05]; p=0·06). Fistula (any grade) occurred in 32 (15%) of 220 patients in the chemotherapy plus bevacizumab groups (all previously irradiated) versus three (1%) of 220 in the chemotherapy-alone groups (all previously irradiated). Grade 3 fistula developed in 13 (6%) versus one (<1%). No fistulas resulted in surgical emergencies, sepsis, or death.
Interpretation: The benefit conferred by incorporation of bevacizumab is sustained with extended follow-up as evidenced by the overall survival curves remaining separated. After progression while receiving bevacizumab, we did not observe a negative rebound effect (ie, shorter survival after bevacizumab is stopped than after chemotherapy alone is stopped). These findings represent proof-of-concept of the efficacy and tolerability of antiangiogenesis therapy in advanced cervical cancer.
Funding: National Cancer Institute.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Dr(s) Tewari, Brady, and Monk report that their institutions and the Gynecologic Oncology Group received grants from Genentech to conduct this clinical trial. Dr(s). Tewari, Burger, and Monk report that they have participated on an Advisory Board and/or served as a speaker on a Round Table Discussion of Bevacizumab for Genentech. Dr(s) Monk and Thigpen have served on a Genentech Speaker’s Bureau. Dr. Penson reported that he received clinical trial funding from Genentech/Roche. All other co-authors, including Dr(s) Sill, Huang, Ramondetta, Look, Landrum, Oaknin, Reid, Leitao, Michael, DiSaia, Copeland, Creasman, Stehman, Birrer, Waggoner, Moore, and Koh report that they have nothing to disclose.
Figures






Comment in
-
Bevacizumab in cervical cancer: a step forward for survival.Lancet. 2017 Oct 7;390(10103):1626-1628. doi: 10.1016/S0140-6736(17)31965-7. Epub 2017 Jul 27. Lancet. 2017. PMID: 28756904 No abstract available.
References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Small W, Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer. 2017 May 2; [Epub ahead of print] - PubMed
-
- Monk BJ, Tewari KS, Koh WJ. Multimodality therapy for locally advanced cervical carcinoma: state of the art and future directions. J Clin Oncol. 2007;25:2952–65. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

