Structural Basis for Substrate Recognition by the Ankyrin Repeat Domain of Human DHHC17 Palmitoyltransferase
- PMID: 28757145
- PMCID: PMC5599134
- DOI: 10.1016/j.str.2017.06.018
Structural Basis for Substrate Recognition by the Ankyrin Repeat Domain of Human DHHC17 Palmitoyltransferase
Abstract
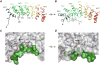
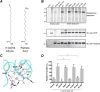
DHHC enzymes catalyze palmitoylation, a major post-translational modification that regulates a number of key cellular processes. There are up to 24 DHHCs in mammals and hundreds of substrate proteins that get palmitoylated. However, how DHHC enzymes engage with their substrates is still poorly understood. There is currently no structural information about the interaction between any DHHC enzyme and protein substrates. In this study we have investigated the structural and thermodynamic bases of interaction between the ankyrin repeat domain of human DHHC17 (ANK17) and Snap25b. We solved a high-resolution crystal structure of the complex between ANK17 and a peptide fragment of Snap25b. Through structure-guided mutagenesis, we discovered key residues in DHHC17 that are critically important for interaction with Snap25b. We further extended our finding by showing that the same residues are also crucial for the interaction of DHHC17 with Huntingtin, one of its most physiologically relevant substrates.
Keywords: DHHC; DHHC17; Huntingtin; Snap25b; X-ray crystallography; palmitoylation; palmitoyltransferase; posttranslational modification; protein lipidation; protein-protein interaction.
Published by Elsevier Ltd.
Figures




Similar articles
-
Identification of a Novel Sequence Motif Recognized by the Ankyrin Repeat Domain of zDHHC17/13 S-Acyltransferases.J Biol Chem. 2015 Sep 4;290(36):21939-50. doi: 10.1074/jbc.M115.657668. Epub 2015 Jul 21. J Biol Chem. 2015. PMID: 26198635 Free PMC article.
-
Peptide array-based screening reveals a large number of proteins interacting with the ankyrin-repeat domain of the zDHHC17 S-acyltransferase.J Biol Chem. 2017 Oct 20;292(42):17190-17202. doi: 10.1074/jbc.M117.799650. Epub 2017 Sep 7. J Biol Chem. 2017. PMID: 28882895 Free PMC article.
-
Multiple palmitoyltransferases are required for palmitoylation-dependent regulation of large conductance calcium- and voltage-activated potassium channels.J Biol Chem. 2010 Jul 30;285(31):23954-62. doi: 10.1074/jbc.M110.137802. Epub 2010 May 27. J Biol Chem. 2010. PMID: 20507996 Free PMC article.
-
Aberrant palmitoylation in Huntington disease.Biochem Soc Trans. 2015 Apr;43(2):205-10. doi: 10.1042/BST20140242. Biochem Soc Trans. 2015. PMID: 25849918 Review.
-
Structure and function of DHHC protein S-acyltransferases.Biochem Soc Trans. 2017 Aug 15;45(4):923-8. doi: 10.1042/BST20160304. Epub 2017 Jun 19. Biochem Soc Trans. 2017. PMID: 28630137 Review.
Cited by
-
Mutant Huntingtin Protein Interaction Map Implicates Dysregulation of Multiple Cellular Pathways in Neurodegeneration of Huntington's Disease.J Huntingtons Dis. 2022;11(3):243-267. doi: 10.3233/JHD-220538. J Huntingtons Dis. 2022. PMID: 35871359 Free PMC article.
-
Structure and Mechanism of DHHC Protein Acyltransferases.J Mol Biol. 2020 Aug 21;432(18):4983-4998. doi: 10.1016/j.jmb.2020.05.023. Epub 2020 Jun 6. J Mol Biol. 2020. PMID: 32522557 Free PMC article. Review.
-
Regulation of Golgi turnover by CALCOCO1-mediated selective autophagy.J Cell Biol. 2021 Jun 7;220(6):e202006128. doi: 10.1083/jcb.202006128. J Cell Biol. 2021. PMID: 33871553 Free PMC article.
-
The Effect of Mutations in the TPR and Ankyrin Families of Alpha Solenoid Repeat Proteins.Front Bioinform. 2021 Jul 6;1:696368. doi: 10.3389/fbinf.2021.696368. eCollection 2021. Front Bioinform. 2021. PMID: 36303725 Free PMC article. Review.
-
Protein lipidation in cancer: mechanisms, dysregulation and emerging drug targets.Nat Rev Cancer. 2024 Apr;24(4):240-260. doi: 10.1038/s41568-024-00666-x. Epub 2024 Feb 29. Nat Rev Cancer. 2024. PMID: 38424304 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

