p53 Mediates Failure of Human Definitive Hematopoiesis in Dyskeratosis Congenita
- PMID: 28757166
- PMCID: PMC5550027
- DOI: 10.1016/j.stemcr.2017.06.015
p53 Mediates Failure of Human Definitive Hematopoiesis in Dyskeratosis Congenita
Abstract
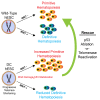
Dyskeratosis congenita (DC) is a bone marrow failure syndrome associated with telomere dysfunction. The progression and molecular determinants of hematopoietic failure in DC remain poorly understood. Here, we use the directed differentiation of human embryonic stem cells harboring clinically relevant mutations in telomerase to understand the consequences of DC-associated mutations on the primitive and definitive hematopoietic programs. Interestingly, telomere shortening does not broadly impair hematopoiesis, as primitive hematopoiesis is not impaired in DC cells. In contrast, while phenotypic definitive hemogenic endothelium is specified, the endothelial-to-hematopoietic transition is impaired in cells with shortened telomeres. This failure is caused by DNA damage accrual and is mediated by p53 stabilization. These observations indicate that detrimental effects of telomere shortening in the hematopoietic system are specific to the definitive hematopoietic lineages. This work illustrates how telomere dysfunction impairs hematopoietic development and creates a robust platform for therapeutic discovery for treatment of DC patients.
Keywords: bone marrow failure; disease modeling; dyskeratosis congenita; embryonic stem cells; hematopoiesis; telomerase; telomere damage; telomeres.
Copyright © 2017 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




References
-
- Bar C., Povedano J.M., Serrano R., Benitez-Buelga C., Popkes M., Formentini I., Bobadilla M., Bosch F., Blasco M.A. Telomerase gene therapy rescues telomere length, bone marrow aplasia, and survival in mice with aplastic anemia. Blood. 2016;127:1770–1779. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

