Focal Adhesion- and IGF1R-Dependent Survival and Migratory Pathways Mediate Tumor Resistance to mTORC1/2 Inhibition
- PMID: 28757207
- PMCID: PMC5698809
- DOI: 10.1016/j.molcel.2017.06.033
Focal Adhesion- and IGF1R-Dependent Survival and Migratory Pathways Mediate Tumor Resistance to mTORC1/2 Inhibition
Abstract
Aberrant signaling by the mammalian target of rapamycin (mTOR) contributes to the devastating features of cancer cells. Thus, mTOR is a critical therapeutic target and catalytic inhibitors are being investigated as anti-cancer drugs. Although mTOR inhibitors initially block cell proliferation, cell viability and migration in some cancer cells are quickly restored. Despite sustained inhibition of mTORC1/2 signaling, Akt, a kinase regulating cell survival and migration, regains phosphorylation at its regulatory sites. Mechanistically, mTORC1/2 inhibition promotes reorganization of integrin/focal adhesion kinase-mediated adhesomes, induction of IGFR/IR-dependent PI3K activation, and Akt phosphorylation via an integrin/FAK/IGFR-dependent process. This resistance mechanism contributes to xenograft tumor cell growth, which is prevented with mTOR plus IGFR inhibitors, supporting this combination as a therapeutic approach for cancers.
Keywords: Akt; dual mTORC1/2 inhibition; mTORC1; mTORC2; tumor resistance.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

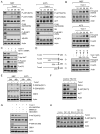



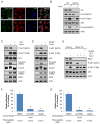

References
-
- Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov. 2011;10:868–880. - PubMed
-
- Bentzinger CF, Romanino K, Cloetta D, Lin S, Mascarenhas JB, Oliveri F, Xia J, Casanova E, Costa CF, Brink M, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424. - PubMed
-
- Braunstein S, Karpisheva K, Pola C, Goldberg J, Hochman T, Yee H, Cangiarella J, Arju R, Formenti SC, Schneider RJ. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell. 2007;28:501–512. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

