Smarcal1-Mediated Fork Reversal Triggers Mre11-Dependent Degradation of Nascent DNA in the Absence of Brca2 and Stable Rad51 Nucleofilaments
- PMID: 28757209
- PMCID: PMC5594205
- DOI: 10.1016/j.molcel.2017.07.001
Smarcal1-Mediated Fork Reversal Triggers Mre11-Dependent Degradation of Nascent DNA in the Absence of Brca2 and Stable Rad51 Nucleofilaments
Abstract
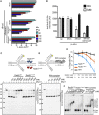
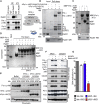
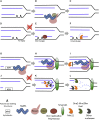
Brca2 deficiency causes Mre11-dependent degradation of nascent DNA at stalled forks, leading to cell lethality. To understand the molecular mechanisms underlying this process, we isolated Xenopus laevis Brca2. We demonstrated that Brca2 protein prevents single-stranded DNA gap accumulation at replication fork junctions and behind them by promoting Rad51 binding to replicating DNA. Without Brca2, forks with persistent gaps are converted by Smarcal1 into reversed forks, triggering extensive Mre11-dependent nascent DNA degradation. Stable Rad51 nucleofilaments, but not RPA or Rad51T131P mutant proteins, directly prevent Mre11-dependent DNA degradation. Mre11 inhibition instead promotes reversed fork accumulation in the absence of Brca2. Rad51 directly interacts with the Pol α N-terminal domain, promoting Pol α and δ binding to stalled replication forks. This interaction likely promotes replication fork restart and gap avoidance. These results indicate that Brca2 and Rad51 prevent formation of abnormal DNA replication intermediates, whose processing by Smarcal1 and Mre11 predisposes to genome instability.
Keywords: Brca2; DNA replication; Mre11; Rad51; Xenopus laevis; fork protection.
Copyright © 2017 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures

Similar articles
-
Deletion of BRCA2 exon 27 causes defects in response to both stalled and collapsed replication forks.Mutat Res. 2014 Aug-Sep;766-767:66-72. doi: 10.1016/j.mrfmmm.2014.06.003. Epub 2014 Jun 22. Mutat Res. 2014. PMID: 25847274
-
Replication fork reversal triggers fork degradation in BRCA2-defective cells.Nat Commun. 2017 Oct 16;8(1):859. doi: 10.1038/s41467-017-01164-5. Nat Commun. 2017. PMID: 29038466 Free PMC article.
-
Brca2, Rad51 and Mre11: performing balancing acts on replication forks.DNA Repair (Amst). 2011 Oct 10;10(10):1060-5. doi: 10.1016/j.dnarep.2011.07.009. Epub 2011 Sep 6. DNA Repair (Amst). 2011. PMID: 21900052 Review.
-
Deletion of BRCA2 exon 27 causes defects in response to both stalled and collapsed replication forks.Mutat Res. 2014 Aug-Sep;766-767:66-72. doi: 10.1016/j.mrfmmm.2014.06.003. Epub 2014 Jun 22. Mutat Res. 2014. PMID: 25773776 Free PMC article.
-
Moonlighting at replication forks - a new life for homologous recombination proteins BRCA1, BRCA2 and RAD51.FEBS Lett. 2017 Apr;591(8):1083-1100. doi: 10.1002/1873-3468.12556. Epub 2017 Jan 30. FEBS Lett. 2017. PMID: 28079255 Review.
Cited by
-
The KU-PARP14 axis differentially regulates DNA resection at stalled replication forks by MRE11 and EXO1.Nat Commun. 2022 Aug 27;13(1):5063. doi: 10.1038/s41467-022-32756-5. Nat Commun. 2022. PMID: 36030235 Free PMC article.
-
ASPM promotes ATR-CHK1 activation and stabilizes stalled replication forks in response to replication stress.Proc Natl Acad Sci U S A. 2022 Oct 4;119(40):e2203783119. doi: 10.1073/pnas.2203783119. Epub 2022 Sep 26. Proc Natl Acad Sci U S A. 2022. PMID: 36161901 Free PMC article.
-
Replication fork uncoupling causes nascent strand degradation and fork reversal.Nat Struct Mol Biol. 2023 Jan;30(1):115-124. doi: 10.1038/s41594-022-00871-y. Epub 2023 Jan 2. Nat Struct Mol Biol. 2023. PMID: 36593312 Free PMC article.
-
Exploiting replication gaps for cancer therapy.Mol Cell. 2022 Jul 7;82(13):2363-2369. doi: 10.1016/j.molcel.2022.04.023. Epub 2022 May 13. Mol Cell. 2022. PMID: 35568026 Free PMC article. Review.
-
Main steps in DNA double-strand break repair: an introduction to homologous recombination and related processes.Chromosoma. 2018 Jun;127(2):187-214. doi: 10.1007/s00412-017-0658-1. Epub 2018 Jan 11. Chromosoma. 2018. PMID: 29327130 Review.
References
-
- Anand R., Ranjha L., Cannavo E., Cejka P. Phosphorylated CtIP functions as a co-factor of the MRE11-RAD50-NBS1 endonuclease in DNA end resection. Mol. Cell. 2016;64:940–950. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

