Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration
- PMID: 28757360
- PMCID: PMC5720381
- DOI: 10.1016/j.stem.2017.07.007
Fusion of Regionally Specified hPSC-Derived Organoids Models Human Brain Development and Interneuron Migration
Abstract
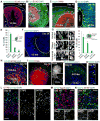
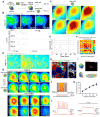
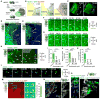
Organoid techniques provide unique platforms to model brain development and neurological disorders. Whereas several methods for recapitulating corticogenesis have been described, a system modeling human medial ganglionic eminence (MGE) development, a critical ventral brain domain producing cortical interneurons and related lineages, has been lacking until recently. Here, we describe the generation of MGE and cortex-specific organoids from human pluripotent stem cells that recapitulate the development of MGE and cortex domains, respectively. Population and single-cell RNA sequencing (RNA-seq) profiling combined with bulk assay for transposase-accessible chromatin with high-throughput sequencing (ATAC-seq) analyses revealed transcriptional and chromatin accessibility dynamics and lineage relationships during MGE and cortical organoid development. Furthermore, MGE and cortical organoids generated physiologically functional neurons and neuronal networks. Finally, fusing region-specific organoids followed by live imaging enabled analysis of human interneuron migration and integration. Together, our study provides a platform for generating domain-specific brain organoids and modeling human interneuron migration and offers deeper insight into molecular dynamics during human brain development.
Keywords: ATAC-seq; MGE; brain organoid; cortex; fusion; hESC; interneuron; neuronal migration; single cell RNA-seq; transcriptional regulation.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




Comment in
-
Putting Two Heads Together to Build a Better Brain.Cell Stem Cell. 2017 Sep 7;21(3):289-290. doi: 10.1016/j.stem.2017.08.017. Cell Stem Cell. 2017. PMID: 28886360
References
-
- Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, Wynshaw-Boris A, Kriegstein AR. Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell. 2017 Jan 9; doi: 10.1016/j.stem.2016.1012.1007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

