The Role of Epigenetic Regulation in Epstein-Barr Virus-Associated Gastric Cancer
- PMID: 28757548
- PMCID: PMC5577998
- DOI: 10.3390/ijms18081606
The Role of Epigenetic Regulation in Epstein-Barr Virus-Associated Gastric Cancer
Abstract
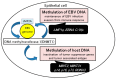
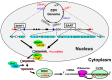
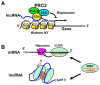
The Epstein-Barr virus (EBV) is detected in about 10% of gastric carcinoma cases throughout the world. In EBV-associated gastric carcinoma (EBVaGC), all tumor cells harbor the clonal EBV genome. The expression of latent EBV genes is strictly regulated through the methylation of EBV DNA. The methylation of viral DNA regulates the type of EBV latency, and methylation of the tumor suppressor genes is a key abnormality in EBVaGC. The methylation frequencies of several tumor suppressor genes and cell adhesion molecules are significantly higher in EBVaGC than in control cases. EBV-derived microRNAs repress translation from viral and host mRNAs. EBV regulates the expression of non-coding RNA in gastric carcinoma. With regard to the clinical application of demethylating agents against EBVaGC, we investigated the effects of decitabine against the EBVaGC cell lines. Decitabine inhibited the cell growth of EBVaGC cells. The promoter regions of p73 and Runt-related transcription factor 3(RUNX3) were demethylated, and their expression was upregulated by the treatment. We review the role of epigenetic regulation in the development and maintenance of EBVaGC and discuss the therapeutic application of DNA demethylating agents for EBVaGC.
Keywords: DNA methylation; demethylating agent; epigenetics; epstein-barr virus; gastric cancer; microRNA; non-coding RNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Burke A.P., Yen T.S., Shekitka K.M., Sobin L.H. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod. Pathol. 1990;3:377–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

