Multifunctional Cinnamic Acid Derivatives
- PMID: 28757554
- PMCID: PMC6152057
- DOI: 10.3390/molecules22081247
Multifunctional Cinnamic Acid Derivatives
Abstract
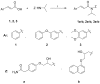
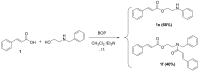
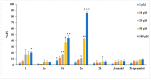
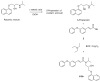
Our research to discover potential new multitarget agents led to the synthesis of 10 novel derivatives of cinnamic acids and propranolol, atenolol, 1-adamantanol, naphth-1-ol, and (benzylamino) ethan-1-ol. The synthesized molecules were evaluated as trypsin, lipoxygenase and lipid peroxidation inhibitors and for their cytotoxicity. Compound 2b derived from phenoxyphenyl cinnamic acid and propranolol showed the highest lipoxygenase (LOX) inhibition (IC50 = 6 μΜ) and antiproteolytic activity (IC50 = 0.425 μΜ). The conjugate 1a of simple cinnamic acid with propranolol showed the higher antiproteolytic activity (IC50 = 0.315 μΜ) and good LOX inhibitory activity (IC50 = 66 μΜ). Compounds 3a and 3b, derived from methoxylated caffeic acid present a promising combination of in vitro inhibitory and antioxidative activities. The S isomer of 2b also presented an interesting multitarget biological profile in vitro. Molecular docking studies point to the fact that the theoretical results for LOX-inhibitor binding are identical to those from preliminary in vitro study.
Keywords: antiproteolytic activity; cinnamic acids; lipoxygenase inhibitors; multitarget.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Multitarget molecular hybrids of cinnamic acids.Molecules. 2014 Dec 2;19(12):20197-226. doi: 10.3390/molecules191220197. Molecules. 2014. PMID: 25474291 Free PMC article.
-
Synthesis and Molecular Modeling of Antioxidant and Anti-Inflammatory Five-Membered Heterocycle-Cinnamic Acid Hybrids.Molecules. 2025 Jul 27;30(15):3148. doi: 10.3390/molecules30153148. Molecules. 2025. PMID: 40807323 Free PMC article.
-
Novel cinnamic acid derivatives as antioxidant and anticancer agents: design, synthesis and modeling studies.Molecules. 2014 Jul 7;19(7):9655-74. doi: 10.3390/molecules19079655. Molecules. 2014. PMID: 25004073 Free PMC article.
-
Application of cinnamic acid in the structural modification of natural products: A review.Phytochemistry. 2023 Feb;206:113532. doi: 10.1016/j.phytochem.2022.113532. Epub 2022 Dec 5. Phytochemistry. 2023. PMID: 36470328 Review.
-
Biological Properties, Health Benefits and Enzymatic Modifications of Dietary Methoxylated Derivatives of Cinnamic Acid.Foods. 2021 Jun 18;10(6):1417. doi: 10.3390/foods10061417. Foods. 2021. PMID: 34207377 Free PMC article. Review.
Cited by
-
Natural product-based radiopharmaceuticals: Focus on curcumin and its analogs, flavonoids, and marine peptides.J Pharm Anal. 2022 Jun;12(3):380-393. doi: 10.1016/j.jpha.2021.07.006. Epub 2021 Jul 21. J Pharm Anal. 2022. PMID: 35811617 Free PMC article. Review.
-
5-(4H)-Oxazolones and Their Benzamides as Potential Bioactive Small Molecules.Molecules. 2020 Jul 11;25(14):3173. doi: 10.3390/molecules25143173. Molecules. 2020. PMID: 32664550 Free PMC article.
-
Cinnamic Acid Conjugates in the Rescuing and Repurposing of Classical Antimalarial Drugs.Molecules. 2019 Dec 24;25(1):66. doi: 10.3390/molecules25010066. Molecules. 2019. PMID: 31878190 Free PMC article. Review.
-
Intensified green-based extraction process as a circular economy approach to recover bioactive compounds from soursop seeds (Annona muricata L.).Food Chem X. 2021 Nov 14;12:100164. doi: 10.1016/j.fochx.2021.100164. eCollection 2021 Dec 30. Food Chem X. 2021. PMID: 35024607 Free PMC article.
-
Pasture-finishing of cattle in Western U.S. rangelands improves markers of animal metabolic health and nutritional compounds in beef.Sci Rep. 2024 Aug 30;14(1):20240. doi: 10.1038/s41598-024-71073-3. Sci Rep. 2024. PMID: 39215122 Free PMC article.
References
-
- Pontiki E., Hadjipavlou-Litina D., Geromichalos G., Papageorgiou A. Anticancer activity and quantitative-structure activity relationship (QSAR) studies of a series of antioxidant/anti-inflammatory aryl-acetic and hydroxamic acids. Chem. Biol. Drug Des. 2009;74:266–275. doi: 10.1111/j.1747-0285.2009.00864.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

