Finding a Balance between Protection and Pathology: The Dual Role of Perforin in Human Disease
- PMID: 28757574
- PMCID: PMC5578000
- DOI: 10.3390/ijms18081608
Finding a Balance between Protection and Pathology: The Dual Role of Perforin in Human Disease
Abstract
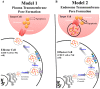
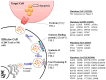
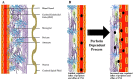
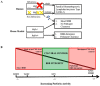
Perforin is critical for controlling viral infection and tumor surveillance. Clinically, mutations in perforin are viewed as unfavorable, as lack of this pore-forming protein results in lethal, childhood disease, familial hemophagocytic lymphohistiocytosis type 2 (FHL 2). However, many mutations in the coding region of PRF1 are not yet associated with disease. Animal models of viral-associated blood-brain barrier (BBB) disruption and experimental cerebral malaria (ECM) have identified perforin as critical for inducing pathologic central nervous system CNS vascular permeability. This review focuses on the role of perforin in both protecting and promoting human disease. It concludes with a novel hypothesis that diversity observed in the PRF1 gene may be an example of selective advantage that protects an individual from perforin-mediated pathology, such as BBB disruption.
Keywords: blood–brain barrier disruption; familial hemophagocytic lymphohistiocytosis type 2; perforin; selective advantage; single nucleotide variants.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Human perforin gene variation is geographically distributed.Mol Genet Genomic Med. 2018 Jan;6(1):44-55. doi: 10.1002/mgg3.344. Epub 2017 Dec 7. Mol Genet Genomic Med. 2018. PMID: 29216683 Free PMC article.
-
Modulatory effects of perforin gene dosage on pathogen-associated blood-brain barrier (BBB) disruption.J Neuroinflammation. 2016 Aug 31;13(1):222. doi: 10.1186/s12974-016-0673-9. J Neuroinflammation. 2016. PMID: 27576583 Free PMC article.
-
Perforin gene mutation in familial haemophagocytic lymphohistiocytosis: the first reported case from Hong Kong.Hong Kong Med J. 2014 Aug;20(4):339-42. doi: 10.12809/hkmj134041. Hong Kong Med J. 2014. PMID: 25104007
-
Associations between PRF1 Ala91Val polymorphism and risk of hemophagocytic lymphohistiocytosis: a meta-analysis based on 1366 subjects.World J Pediatr. 2020 Dec;16(6):598-606. doi: 10.1007/s12519-020-00351-7. Epub 2020 Mar 20. World J Pediatr. 2020. PMID: 32198610 Review.
-
Angeborene hämophagozytische Lymphohistiozytose (HLH).Klin Padiatr. 2010 Nov;222(6):345-50. doi: 10.1055/s-0029-1246165. Epub 2010 May 10. Klin Padiatr. 2010. PMID: 20458667 Review.
Cited by
-
Targeting of Perforin Inhibitor into the Brain Parenchyma Via a Prodrug Approach Can Decrease Oxidative Stress and Neuroinflammation and Improve Cell Survival.Mol Neurobiol. 2020 Nov;57(11):4563-4577. doi: 10.1007/s12035-020-02045-7. Epub 2020 Aug 5. Mol Neurobiol. 2020. PMID: 32754897 Free PMC article.
-
The pore conformation of lymphocyte perforin.Sci Adv. 2022 Feb 11;8(6):eabk3147. doi: 10.1126/sciadv.abk3147. Epub 2022 Feb 11. Sci Adv. 2022. PMID: 35148176 Free PMC article.
-
Perforin 1 in Cancer: Mechanisms, Therapy, and Outlook.Biomolecules. 2024 Jul 26;14(8):910. doi: 10.3390/biom14080910. Biomolecules. 2024. PMID: 39199299 Free PMC article. Review.
-
Dual role of CXCL10 in cancer progression: implications for immunotherapy and targeted treatment.Cancer Biol Ther. 2025 Dec;26(1):2538962. doi: 10.1080/15384047.2025.2538962. Epub 2025 Aug 4. Cancer Biol Ther. 2025. PMID: 40760734 Free PMC article. Review.
-
IL7R, GZMA and CD8A serve as potential molecular biomarkers for sepsis based on bioinformatics analysis.Front Immunol. 2024 Nov 25;15:1445858. doi: 10.3389/fimmu.2024.1445858. eCollection 2024. Front Immunol. 2024. PMID: 39654893 Free PMC article.
References
-
- Lopez J.A., Susanto O., Jenkins M.R., Lukoyanova N., Sutton V.R., Law R.H., Johnston A., Bird C.H., Bird P.I., Whisstock J.C., et al. Perforin forms transient pores on the target cell plasma membrane to facilitate rapid access of granzymes during killer cell attack. Blood. 2013;121:2659–2668. doi: 10.1182/blood-2012-07-446146. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

