Microbubbles-Assisted Ultrasound Triggers the Release of Extracellular Vesicles
- PMID: 28757579
- PMCID: PMC5578002
- DOI: 10.3390/ijms18081610
Microbubbles-Assisted Ultrasound Triggers the Release of Extracellular Vesicles
Abstract
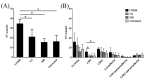
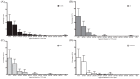
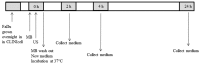
Microbubbles-assisted ultrasound (USMB) has shown promise in improving local drug delivery. The formation of transient membrane pores and endocytosis are reported to be enhanced by USMB, and they contribute to cellular drug uptake. Exocytosis also seems to be linked to endocytosis upon USMB treatment. Based on this rationale, we investigated whether USMB triggers exocytosis resulting in the release of extracellular vesicles (EVs). USMB was performed on a monolayer of head-and-neck cancer cells (FaDu) with clinically approved microbubbles and commonly used ultrasound parameters. At 2, 4, and 24 h, cells and EV-containing conditioned media from USMB and control conditions (untreated cells, cells treated with microbubbles and ultrasound only) were harvested. EVs were measured using flow cytometric immuno-magnetic bead capture assay, immunogold electron microscopy, and western blotting. After USMB, levels of CD9 exposing-EVs significantly increased at 2 and 4 h, whereas levels of CD63 exposing-EVs increased at 2 h. At 24 h, EV levels were comparable to control levels. EVs released after USMB displayed a heterogeneous size distribution profile (30-1200 nm). Typical EV markers CD9, CD63, and alix were enriched in EVs released from USMB-treated FaDu cells. In conclusion, USMB treatment triggers exocytosis leading to the release of EVs from FaDu cells.
Keywords: drug delivery; endocytosis; exocytosis; exosomes; microvesicles; sonoporation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Ultrasound and microbubble induced release from intracellular compartments.BMC Biotechnol. 2017 May 18;17(1):45. doi: 10.1186/s12896-017-0364-3. BMC Biotechnol. 2017. PMID: 28521780 Free PMC article.
-
Potential Use of Extracellular Vesicles Generated by Microbubble-Assisted Ultrasound as Drug Nanocarriers for Cancer Treatment.Int J Mol Sci. 2020 Apr 24;21(8):3024. doi: 10.3390/ijms21083024. Int J Mol Sci. 2020. PMID: 32344752 Free PMC article.
-
Ultrasound Microbubble Treatment Enhances Clathrin-Mediated Endocytosis and Fluid-Phase Uptake through Distinct Mechanisms.PLoS One. 2016 Jun 8;11(6):e0156754. doi: 10.1371/journal.pone.0156754. eCollection 2016. PLoS One. 2016. PMID: 27275866 Free PMC article.
-
Extracellular vesicles from activated platelets: a semiquantitative cryo-electron microscopy and immuno-gold labeling study.Platelets. 2017 May;28(3):263-271. doi: 10.1080/09537104.2016.1268255. Epub 2017 Jan 19. Platelets. 2017. PMID: 28102751 Review.
-
Engineered extracellular vesicles and their mimetics for clinical translation.Methods. 2020 May 1;177:80-94. doi: 10.1016/j.ymeth.2019.10.005. Epub 2019 Oct 15. Methods. 2020. PMID: 31626895 Review.
Cited by
-
Landscape of Cellular Bioeffects Triggered by Ultrasound-Induced Sonoporation.Int J Mol Sci. 2022 Sep 23;23(19):11222. doi: 10.3390/ijms231911222. Int J Mol Sci. 2022. PMID: 36232532 Free PMC article. Review.
-
Focused Ultrasound Hyperthermia Augments Release of Glioma-derived Extracellular Vesicles with Differential Immunomodulatory Capacity.Theranostics. 2020 Jun 12;10(16):7436-7447. doi: 10.7150/thno.46534. eCollection 2020. Theranostics. 2020. PMID: 32642004 Free PMC article.
-
Applications of Ultrasound-Mediated Gene Delivery in Regenerative Medicine.Bioengineering (Basel). 2022 Apr 27;9(5):190. doi: 10.3390/bioengineering9050190. Bioengineering (Basel). 2022. PMID: 35621468 Free PMC article. Review.
-
Functional intersections between extracellular vesicles and oncolytic therapies.Trends Pharmacol Sci. 2021 Nov;42(11):883-896. doi: 10.1016/j.tips.2021.09.001. Epub 2021 Sep 28. Trends Pharmacol Sci. 2021. PMID: 34598797 Free PMC article. Review.
-
Influence of cell shape on sonoporation efficiency in microbubble-facilitated delivery using micropatterned cell arrays.Sci Rep. 2024 Dec 28;14(1):30845. doi: 10.1038/s41598-024-81410-1. Sci Rep. 2024. PMID: 39730459 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

