Role of Mitochondria and Endoplasmic Reticulum in Taurine-Deficiency-Mediated Apoptosis
- PMID: 28757580
- PMCID: PMC5579589
- DOI: 10.3390/nu9080795
Role of Mitochondria and Endoplasmic Reticulum in Taurine-Deficiency-Mediated Apoptosis
Abstract
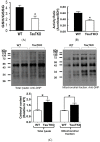
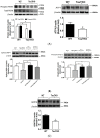
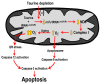
Taurine is a ubiquitous sulfur-containing amino acid found in high concentration in most tissues. Because of its involvement in fundamental physiological functions, such as regulating respiratory chain activity, modulating cation transport, controlling inflammation, altering protein phosphorylation and prolonging lifespan, taurine is an important nutrient whose deficiency leads to severe pathology and cell death. However, the mechanism by which taurine deficiency causes cell death is inadequately understood. Therefore, the present study examined the hypothesis that overproduction of reactive oxygen species (ROS) by complex I of the respiratory chain triggers mitochondria-dependent apoptosis in hearts of taurine transporter knockout (TauTKO) mice. In support of the hypothesis, a 60% decrease in mitochondrial taurine content of 3-month-old TauTKO hearts was observed, which was associated with diminished complex I activity and the onset of mitochondrial oxidative stress. Oxidative damage to stressed mitochondria led to activation of a caspase cascade, with stimulation of caspases 9 and 3 prevented by treatment of 3-month-old TauTKO mice with the mitochondria specific antioxidant, MitoTempo. In 12 month-old, but not 3-month-old, TauTKO hearts, caspase 12 activation contributes to cell death, revealing a pathological role for endoplasmic reticulum (ER) stress in taurine deficient, aging mice. Thus, taurine is a cytoprotective nutrient that ensures normal mitochondrial and ER function, which is important for the reduction of risk for apoptosis and premature death.
Keywords: apoptosis; caspase cascade; endoplasmic reticulum stress; mitochondria; mitochondria encoded proteins; oxidative stress; respiratory chain; tRNALeu(UUR).
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
The ubiquitin-proteasome system and autophagy are defective in the taurine-deficient heart.Amino Acids. 2015 Dec;47(12):2609-22. doi: 10.1007/s00726-015-2053-7. Epub 2015 Jul 21. Amino Acids. 2015. PMID: 26193770
-
Deficiency in the mitochondrial apoptotic pathway reveals the toxic potential of autophagy under ER stress conditions.Autophagy. 2014;10(11):1921-36. doi: 10.4161/15548627.2014.981790. Autophagy. 2014. PMID: 25470234 Free PMC article.
-
Taurine depletion caused by knocking out the taurine transporter gene leads to cardiomyopathy with cardiac atrophy.J Mol Cell Cardiol. 2008 May;44(5):927-37. doi: 10.1016/j.yjmcc.2008.03.001. Epub 2008 Mar 18. J Mol Cell Cardiol. 2008. PMID: 18407290
-
Role of antioxidant activity of taurine in diabetes.Can J Physiol Pharmacol. 2009 Feb;87(2):91-9. doi: 10.1139/Y08-110. Can J Physiol Pharmacol. 2009. PMID: 19234572 Review.
-
Cardiac and skeletal muscle abnormality in taurine transporter-knockout mice.J Biomed Sci. 2010 Aug 24;17 Suppl 1(Suppl 1):S20. doi: 10.1186/1423-0127-17-S1-S20. J Biomed Sci. 2010. PMID: 20804595 Free PMC article. Review.
Cited by
-
Metabolomic analysis of serum and myocardium in compensated heart failure after myocardial infarction.Life Sci. 2019 Mar 15;221:212-223. doi: 10.1016/j.lfs.2019.01.040. Epub 2019 Feb 5. Life Sci. 2019. PMID: 30731143 Free PMC article.
-
Taurine Supplementation Alleviates Puromycin Aminonucleoside Damage by Modulating Endoplasmic Reticulum Stress and Mitochondrial-Related Apoptosis in Rat Kidney.Nutrients. 2018 May 29;10(6):689. doi: 10.3390/nu10060689. Nutrients. 2018. PMID: 29843457 Free PMC article.
-
A plasma metabolomic signature of Leber hereditary optic neuropathy showing taurine and nicotinamide deficiencies.Hum Mol Genet. 2021 Mar 25;30(1):21-29. doi: 10.1093/hmg/ddab013. Hum Mol Genet. 2021. PMID: 33437983 Free PMC article.
-
Targeting ER stress and calpain activation to reverse age-dependent mitochondrial damage in the heart.Mech Ageing Dev. 2020 Dec;192:111380. doi: 10.1016/j.mad.2020.111380. Epub 2020 Oct 9. Mech Ageing Dev. 2020. PMID: 33045249 Free PMC article. Review.
-
Taurine and its analogs in neurological disorders: Focus on therapeutic potential and molecular mechanisms.Redox Biol. 2019 Jun;24:101223. doi: 10.1016/j.redox.2019.101223. Epub 2019 May 21. Redox Biol. 2019. PMID: 31141786 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

