Chalcone Derivatives: Promising Starting Points for Drug Design
- PMID: 28757583
- PMCID: PMC6152227
- DOI: 10.3390/molecules22081210
Chalcone Derivatives: Promising Starting Points for Drug Design
Abstract
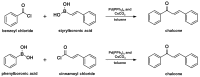
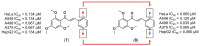
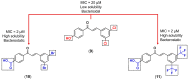
Medicinal chemists continue to be fascinated by chalcone derivatives because of their simple chemistry, ease of hydrogen atom manipulation, straightforward synthesis, and a variety of promising biological activities. However, chalcones have still not garnered deserved attention, especially considering their high potential as chemical sources for designing and developing new effective drugs. In this review, we summarize current methodological developments towards the design and synthesis of new chalcone derivatives and state-of-the-art medicinal chemistry strategies (bioisosterism, molecular hybridization, and pro-drug design). We also highlight the applicability of computer-assisted drug design approaches to chalcones and address how this may contribute to optimizing research outputs and lead to more successful and cost-effective drug discovery endeavors. Lastly, we present successful examples of the use of chalcones and suggest possible solutions to existing limitations.
Keywords: chalcone derivatives; chalcone synthesis; computer-assisted drug design; molecular modification strategies; natural products.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
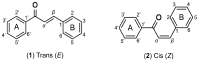
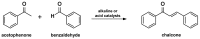
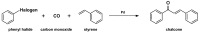
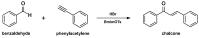
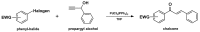
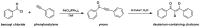
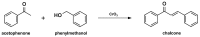
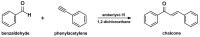
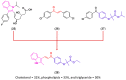
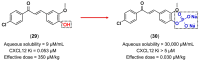
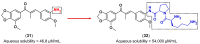
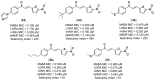
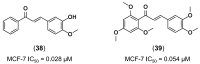
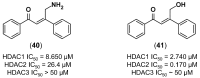
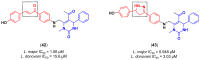
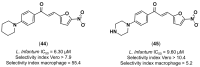
References
-
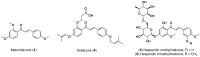
- Ni L., Meng C.Q., Sikorski J.A. Recent advances in therapeutic chalcones. Expert Opin. Ther. Pat. 2004;14:1669–1691. doi: 10.1517/13543776.14.12.1669. - DOI
-
- Wong E. The role of chalcones and flavanones in flavonoid biosynthesis. Phytochemistry. 1968;7:1751–1758. doi: 10.1016/S0031-9422(00)86646-7. - DOI
-
- Evranos Aksöz B., Ertan R. Chemical and structural properties of chalcones I. FABAD J. Pharm. Sci. 2011;36:223–242.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

