2,2'-Bipyridyl formation from 2-arylpyridines through bimetallic diyttrium intermediate
- PMID: 28757942
- PMCID: PMC5510530
- DOI: 10.1039/c5sc01599e
2,2'-Bipyridyl formation from 2-arylpyridines through bimetallic diyttrium intermediate
Abstract
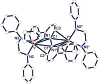
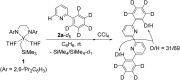
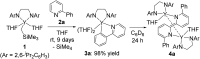
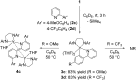
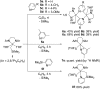
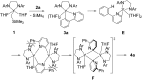
An alkylyttrium complex supported by an N,N'-bis(2,6-diisopropylphenyl)ethylenediamido ligand, (ArNCH2CH2NAr)Y(CH2SiMe3)(THF)2 (1, Ar = 2,6- i Pr2C6H3), activated an ortho-phenyl C-H bond of 2-phenylpyridine (2a) to form a (2-pyridylphenyl)yttrium complex (3a) containing a five-membered metallacycle. Subsequently, a unique C(sp2)-C(sp2) coupling of 2-phenylpyridine proceeded through a bimetallic yttrium intermediate, derived from an intramolecular shift of the yttrium center to an ortho-position of the pyridine ring in 3a, to yield a bimetallic yttrium complex (4a) bridged by two-electron reduced 6,6'-diphenyl-2,2'-bipyridyl. Aryl substituents at the ortho-position of the pyridine ring were key in order to destabilize the μ,κ2-(C,N)-pyridyldiyttrium intermediate prior to the C(sp2)-C(sp2) bond formation.
Figures

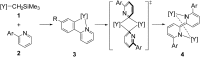

Similar articles
-
Direct ortho-C-H Aminoalkylation of 2-Substituted Pyridine Derivatives Catalyzed by Yttrium Complexes with N,N'-Diarylethylenediamido Ligands.J Am Chem Soc. 2018 Jun 13;140(23):7332-7342. doi: 10.1021/jacs.8b03998. Epub 2018 Jun 5. J Am Chem Soc. 2018. PMID: 29775321
-
Versatile reactivities of rare-earth metal dialkyl complexes supported by a neutral pyrrolyl-functionalized β-diketiminato ligand.Dalton Trans. 2018 Mar 12;47(11):3947-3957. doi: 10.1039/c7dt04410k. Dalton Trans. 2018. PMID: 29459931
-
Hemilabile N-xylyl-N'-methylperimidine carbene iridium complexes as catalysts for C-H activation and dehydrogenative silylation: dual role of N-xylyl moiety for ortho-C-H bond activation and reductive bond cleavage.J Am Chem Soc. 2013 Sep 4;135(35):13149-61. doi: 10.1021/ja406519u. Epub 2013 Aug 23. J Am Chem Soc. 2013. PMID: 23914836
-
Trinuclear alkyl hydrido rare-earth complexes supported by amidopyridinato ligands: synthesis, structures, C-Si bond activation and catalytic activity in ethylene polymerization.Dalton Trans. 2014 Oct 14;43(38):14450-60. doi: 10.1039/c4dt00806e. Dalton Trans. 2014. PMID: 24983231
-
Transition Metal-Catalyzed Regioselective Direct C-H Amidation: Interplay between Inner- and Outer-Sphere Pathways for Nitrene Cross-Coupling Reactions.Acc Chem Res. 2022 Aug 2;55(15):2123-2137. doi: 10.1021/acs.accounts.2c00283. Epub 2022 Jul 19. Acc Chem Res. 2022. PMID: 35853135
Cited by
-
Dehydrogenative coupling of 4-substituted pyridines mediated by a zirconium(ii) synthon: reaction pathways and dead ends.Chem Sci. 2018 May 16;9(23):5223-5232. doi: 10.1039/c8sc01025k. eCollection 2018 Jun 21. Chem Sci. 2018. PMID: 29997877 Free PMC article.
-
Rare-earth metal complexes bearing electrophilic and nucleophilic carbon centres and their unique reactivity patterns towards pyridine derivatives.Chem Sci. 2024 Nov 12;15(48):20315-20327. doi: 10.1039/d4sc04197f. eCollection 2024 Dec 11. Chem Sci. 2024. PMID: 39568940 Free PMC article.
References
-
- Nelson T. D., Crouch R. D. Org. React. 2004;63:265.
- Yamamoto Y., Copper-mediated aryl-aryl bond formation leading to biaryls. A century after the Ullmann breakthrough, in Copper-Mediated Cross-Coupling Reactions, ed. G. Evano and N. Blanchard, John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014, p. 335.
-
- Bringmann G., Walter R., Weirich R. Angew. Chem., Int. Ed. Engl. 1990;29:977.
- Hassan J., Sevignon M., Gozzi C., Schulz E., Lemaire M. Chem. Rev. 2002;102:1359. - PubMed
-
-
For recent reviews of dehydrogenative coupling reactions, see:
- Yeung C. S., Dong V. M. Chem. Rev. 2011;111:1215. - PubMed
- Sun C.-L., Li B.-J., Shi Z.-J. Chem. Rev. 2011;111:1293. - PubMed
- Liu C., Zhang H., Shi W., Lei A. Chem. Rev. 2011;111:1780. - PubMed
- Cho S. H., Kim J. Y., Kwak J., Chang S. Chem. Soc. Rev. 2011;40:5068. - PubMed
- Ashenhurst J. A. Chem. Soc. Rev. 2010;39:540. - PubMed
- Ackermann L., Vicente R., Kapdi A. R., Angew. Chem., Int. Ed., 2009, 48 , 9792 , . For recent reports of dehydrogenative 2,2′-bipyridine formation, see . - PubMed
- Kawashima T., Takao T., Suzuki H. J. Am. Chem. Soc. 2007;129:11006. - PubMed
- Takao T., Kawashima T., Kanda H., Okamura R., Suzuki H. Organometallics. 2012;31:4817.
-
-
- Nakamura A., Otsuka S. Tetrahedron Lett. 1974;15:463.
- Tsou T. T., Kochi J. K. J. Am. Chem. Soc. 1979;101:7547.
-
-
For reports of biaryl formation from phenyllanthanide complexes, see:
- Fryzuk M. D., Love J. B., Rettig S. J. J. Am. Chem. Soc. 1997;119:9071.
- Fryzuk M. D., Jafarpour L., Kerton F. M., Love J. B., Patrick B. O., Rettig S. J. Organometallics. 2001;20:1387.
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

