A highly convergent synthesis of the C1-C31 polyol domain of amphidinol 3 featuring a TST-RCM reaction: confirmation of the revised relative stereochemistry
- PMID: 28757956
- PMCID: PMC5507186
- DOI: 10.1039/c5sc00814j
A highly convergent synthesis of the C1-C31 polyol domain of amphidinol 3 featuring a TST-RCM reaction: confirmation of the revised relative stereochemistry
Abstract
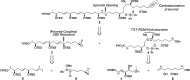
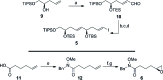
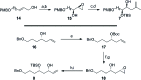
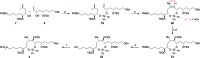
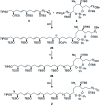
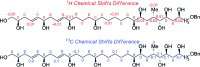
The concise enantioselective synthesis of the revised C1-C31 fragment of the polyketide amphidinol 3 was accomplished in 16 steps and 12.8% overall yield. Salient features of the strategy include chemoselective Weinreb amide coupling and concomitant CBS reduction for the preparation of the C1-C15 tris-syn-1,5-diol motif and a temporary silicon-tethered ring-closing metathesis (TST-RCM) reaction in combination with a diastereoselective hydroboration for the construction of the C16-C31 polypropionate fragment. The union of the fragments was accomplished by a regioselective ring-opening of the terminal epoxide with a phenyl sulfone stabilized carbanion, which upon reduction and deprotection permits a comparison of the relative configuration with the natural product.
Figures



References
-
-
For isolation, structural characterization and bioactivity evaluation of the amphidinol family, see:
- Satake M., Murata M., Yasumoto T., Fujita T., Naoki H. J. Am. Chem. Soc. 1991;113:9859.
- Paul G. K., Matsumori N., Murata M., Tachibana K. Tetrahedron Lett. 1995;36:6279.
- Paul G. K., Matsumori N., Konoki K., Sasaki M., Murata M. and Tachibana K., in Harmful and Toxic Algal Blooms, Proceedings of the Seventh International Conference on Toxic Phytoplankton, ed. T. Yasumoto, Y. Oshima and Y. Fukuyo, UNESCO, Sendai, Japan, 1996, p. 503.
- Paul G. K., Matsumori N., Konoki K., Murata M., Tachibana K. J. Mar. Biotechnol. 1997;5:124.
- Echigoya R., Rhodes L., Oshima Y., Satake M. Harmful Algae. 2005;4:383.
- Morsy N., Matsuoka S., Houdai T., Matsumori N., Adachi S., Murata M., Iwashita T., Fujita T. Tetrahedron. 2005;61:8606.
- Morsy N., Houdai T., Matsuoka S., Matsumori N., Adachi S., Oishi T., Murata M., Iwashita T., Fujita T. Bioorg. Med. Chem. 2006;14:6548. - PubMed
- Meng Y., Van Wagoner R. M., Misner I., Tomas C., Wright J. L. C. J. Nat. Prod. 2010;73:409. - PubMed
- Nuzzo G., Cutignano A., Sardo A., Fontana A., J. Nat. Prod., 2014, 77 , 1524 , and pertinent references therein . - PubMed
-
-
- For a recent report that proposes a barrel-stave pore model, see: Espiritu R. A., Matsumori N., Tsuda M., Murata M., Biochemistry, 2014, 53 , 3287 , and pertinent references cited therein . - PubMed
-
- For the original structural elucidation of amphidinol 3 (1), see: Murata M., Matsuoka S., Matsumori N., Paul G. K., Tachibana K., J. Am. Chem. Soc., 1999, 121 , 870 .
-
-
For structural revision of amphidinol 3 (1), see:
- Oishi T., Kanemoto M., Swasono R., Matsumori N., Murata M. Org. Lett. 2008;10:5203. - PubMed
- Swasono R. T., Kanemoto M., Matsumori N., Oishi T., Murata M. Heterocycles. 2011;82:1359.
- Manabe Y., Ebine M., Matsumori N., Murata M., Oishi T. J. Nat. Prod. 2012;75:2003. - PubMed
- Ebine M., Kanemoto M., Manabe Y., Konno Y., Sakai K., Matsumori N., Murata M., Oishi T. Org. Lett. 2013;15:2846. - PubMed
-
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

