Multistep energy and electron transfer processes in novel rotaxane donor-acceptor hybrids generating microsecond-lived charge separated states
- PMID: 28757988
- PMCID: PMC5512142
- DOI: 10.1039/c5sc02895g
Multistep energy and electron transfer processes in novel rotaxane donor-acceptor hybrids generating microsecond-lived charge separated states
Abstract
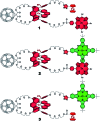
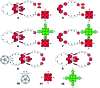
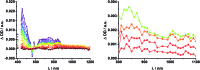
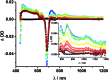
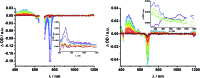
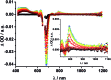
A new set of [Cu(phen)2]+ based rotaxanes, featuring [60]-fullerene as an electron acceptor and a variety of electron donating moieties, namely zinc porphyrin (ZnP), zinc phthalocyanine (ZnPc) and ferrocene (Fc), has been synthesized and fully characterized with respect to electrochemical and photophysical properties. The assembly of the rotaxanes has been achieved using a slight variation of our previously reported synthetic strategy that combines the Cu(i)-catalyzed azide-alkyne cycloaddition reaction (the "click" or CuAAC reaction) with Sauvage's metal-template protocol. To underline our results, complementary model rotaxanes and catenanes have been prepared using the same strategy and their electrochemistry and photo-induced processes have been investigated. Insights into excited state interactions have been afforded from steady state and time resolved emission spectroscopy as well as transient absorption spectroscopy. It has been found that photo-excitation of the present rotaxanes triggers a cascade of multi-step energy and electron transfer events that ultimately leads to remarkably long-lived charge separated states featuring one-electron reduced C60 radical anion (C60˙-) and either one-electron oxidized porphyrin (ZnP˙+) or one-electron oxidized ferrocene (Fc˙+) with lifetimes up to 61 microseconds. In addition, shorter-lived charge separated states involving one-electron oxidized copper complexes ([Cu(phen)2]2+ (τ < 100 ns)), one-electron oxidized zinc phthalocyanine (ZnPc˙+; τ = 380-560 ns), or ZnP˙+ (τ = 2.3-8.4 μs), and C60˙- have been identified as intermediates during the sequence. Detailed energy diagrams illustrate the sequence and rate constants of the photophysical events occurring with the mechanically-linked chromophores. This work pioneers the exploration of mechanically-linked systems as platforms to position three distinct chromophores, which are able to absorb light over a very wide range of the visible region, triggering a cascade of short-range energy and electron transfer processes to afford long-lived charge separated states.
Figures










Similar articles
-
Synthesis and photophysical properties of new catenated electron donor-acceptor materials with magnesium and free base porphyrins as donors and C60 as the acceptor.Nanoscale. 2015 Jan 21;7(3):1145-60. doi: 10.1039/c4nr06146b. Nanoscale. 2015. PMID: 25482308
-
Topological and Conformational Effects on Electron Transfer Dynamics in Porphyrin-[60]Fullerene Interlocked Systems.Chem Mater. 2012 Jul 10;24(13):2472-2485. doi: 10.1021/cm3004408. Epub 2012 Jun 18. Chem Mater. 2012. PMID: 22984324 Free PMC article.
-
Design, synthesis and photoinduced processes in molecular interlocked photosynthetic [60]fullerene systems.Chem Soc Rev. 2020 Jan 2;49(1):8-20. doi: 10.1039/c9cs00638a. Chem Soc Rev. 2020. PMID: 31808480
-
[2]Catenanes decorated with porphyrin and [60]fullerene groups: design, convergent synthesis, and photoinduced processes.J Am Chem Soc. 2010 Mar 24;132(11):3847-61. doi: 10.1021/ja910149f. J Am Chem Soc. 2010. PMID: 20196597 Free PMC article.
-
Porphyrin-fullerene linked systems as artificial photosynthetic mimics.Org Biomol Chem. 2004 May 21;2(10):1425-33. doi: 10.1039/b403024a. Epub 2004 Apr 26. Org Biomol Chem. 2004. PMID: 15136797 Review.
Cited by
-
The impact of aggregation of AIE and ACQ moiety-integrating material on the excited state dynamics.RSC Adv. 2023 Nov 20;13(48):33911-33917. doi: 10.1039/d3ra06359c. eCollection 2023 Nov 16. RSC Adv. 2023. PMID: 38020029 Free PMC article.
-
Synergy of Electrostatic and π-π Interactions in the Realization of Nanoscale Artificial Photosynthetic Model Systems.Angew Chem Int Ed Engl. 2020 Oct 12;59(42):18786-18794. doi: 10.1002/anie.202006014. Epub 2020 Sep 2. Angew Chem Int Ed Engl. 2020. PMID: 32652750 Free PMC article.
-
Ru(II)Porphyrinate-based molecular nanoreactor for carbene insertion reactions and quantitative formation of rotaxanes by active-metal-template syntheses.Nat Commun. 2020 Dec 11;11(1):6370. doi: 10.1038/s41467-020-20046-x. Nat Commun. 2020. PMID: 33311502 Free PMC article.
-
Ultrafast Dynamics of Charge Transfer and Photochemical Reactions in Solar Energy Conversion.Adv Sci (Weinh). 2018 Oct 11;5(12):1800221. doi: 10.1002/advs.201800221. eCollection 2018 Dec. Adv Sci (Weinh). 2018. PMID: 30581691 Free PMC article. Review.
-
Selective photoinduced charge separation in perylenediimide-pillar[5]arene rotaxanes.Nat Commun. 2022 Jan 20;13(1):415. doi: 10.1038/s41467-022-28022-3. Nat Commun. 2022. PMID: 35058440 Free PMC article.
References
-
- Kirner S., Sekita M., Guldi D. M. Adv. Mater. 2014;26:1482–1493. - PubMed
-
- Anelli P. L., Ashton P. R., Ballardini R., Balzani V., Delgado M., Gandolfi M. T., Goodnow T. T., Kaifer A. E., Philp D., Marek P., Prodi L., Reddington M. V., Slawin A. M. Z., Spencer N., Stoddart J. F., Vicent C., Williams D. J. J. Am. Chem. Soc. 1992;114:193–218.
-
- Ashton P. R., Balzani V., Credi A., Kocian O., Pasini D., Prodi L., Spencer N., Stoddart J. F., Tolley M. S., Venturi M., White A. J. P., Williams D. J. Chem.–Eur. J. 1998;4:590–607.
-
- Ashton P. R., Goodnow T. T., Kaifer A. E., Reddington M. V., Slawin A. M. Z., Spencer N., Stoddart J. F., Vicent C., Williams D. J. Angew. Chem., Int. Ed. 1989;28:1396–1399.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

