Vitrimers: permanent organic networks with glass-like fluidity
- PMID: 28757995
- PMCID: PMC5508697
- DOI: 10.1039/c5sc02223a
Vitrimers: permanent organic networks with glass-like fluidity
Abstract
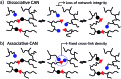
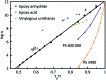
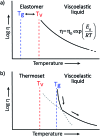
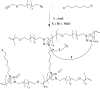
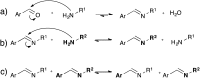
Most covalent adaptable networks give highly interesting properties for material processing such as reshaping, recycling and repairing. Classical thermally reversible chemical cross-links allow for a heat-triggered switch between materials that behave as insoluble cured resins, and liquid thermoplastic materials, through a fully reversible sol-gel transition. In 2011, a new class of materials, coined vitrimers, was introduced, which extended the realm of adaptable organic polymer networks. Such materials have the remarkable property that they can be thermally processed in a liquid state without losing network integrity. This feature renders the materials processable like vitreous glass, not requiring precise temperature control. In this mini-review, an overview of the state-of-the-art in the quickly emerging field of vitrimer materials is presented. With a main focus on the chemical origins of their unique thermal behavior, the existing chemical systems and their properties will be discussed. Furthermore, future prospects and challenges in this important research field are highlighted.
Figures


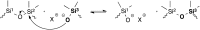





References
-
- Bowman C. N., Kloxin C. J. Angew. Chem., Int. Ed. 2012;51:4272–4274. - PubMed
-
- Kloxin C. J., Bowman C. N. Chem. Soc. Rev. 2013;42:7161–7173. - PubMed
-
- Scott T. F., Bowman C. N., Wayne C. D., Schneider A. D. Science. 2005;308:1615–1617. - PubMed
-
- Kloxin C. J., Scott T. F., Park H. Y., Bowman C. N. Adv. Mater. 2011;23:1977–1981. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

