The Opportunities of Metabolomics in Drug Safety Evaluation
- PMID: 28758057
- PMCID: PMC5526643
- DOI: 10.1007/s40495-016-0079-5
The Opportunities of Metabolomics in Drug Safety Evaluation
Abstract
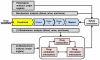
Although safety of drug candidates is carefully monitored in preclinical and clinical studies using a variety of approaches, drug toxicity may still occur in clinical practice. Therefore, novel approaches are needed to complement the current drug safety evaluation system. Metabolomics comprehensively analyzes the metabolites altered by drug exposure, which can therefore be used to profile drug metabolism, endobiotic metabolism, and drug-microbiota interactions. The information from metabolomic analysis can be used to determine the off-targets of a drug candidate, and thus provide a mechanistic understanding of drug toxicity. We herein discuss the opportunities of metabolomics in drug safety evaluation.
Keywords: Metabolomics; drug metabolites; drug safety evaluation; endobiotic metabolites.
Figures
References
-
- Waring MJ, Arrowsmith J, Leach AR, Leeson PD, Mandrell S, Owen RM, et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov. 2015;14:475–86. - PubMed
-
- Fung M, Thornton A, Mybeck K, Wu JH-h, Hornbuckle K, Muniz E. Evaluation of the characteristics of safety withdrawal of prescription drugs from worldwide pharmaceutical markets-1960 to 1999*. Drug Inf J. 2001;35:293–317.
-
- Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. - PubMed
-
- Wishart DS. Applications of metabolomics in drug discovery and development. Drugs in R & D. 2008;9:307–22. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
