Effect of Interactions between Harvester Ants on Forager Decisions
- PMID: 28758093
- PMCID: PMC5531068
- DOI: 10.3389/fevo.2016.00115
Effect of Interactions between Harvester Ants on Forager Decisions
Abstract
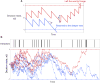
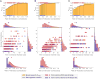
Harvester ant colonies adjust their foraging activity to day-to-day changes in food availability and hour-to-hour changes in environmental conditions. This collective behavior is regulated through interactions, in the form of brief antennal contacts, between outgoing foragers and returning foragers with food. Here we consider how an ant, waiting in the entrance chamber just inside the nest entrance, uses its accumulated experience of interactions to decide whether to leave the nest to forage. Using videos of field observations, we tracked the interactions and foraging decisions of ants in the entrance chamber. Outgoing foragers tended to interact with returning foragers at higher rates than ants that returned to the deeper nest and did not forage. To provide a mechanistic framework for interpreting these results, we develop a decision model in which ants make decisions based upon a noisy accumulation of individual contacts with returning foragers. The model can reproduce core trends and realistic distributions for individual ant interaction statistics, and suggests possible mechanisms by which foraging activity may be regulated at an individual ant level.
Keywords: collective behavior; decision-making; integrator; sequential sampling model; stochastic accumulator.
Conflict of interest statement
Conflict of Interest Statement: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


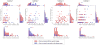



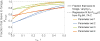
References
-
- Beverly BD, McLendon H, Nacu S, Holmes S, Gordon DM. How site fidelity leads to individual differences in the foraging activity of harvester ants. Behav Ecol. 2009;20:633–638. doi: 10.1093/beheco/arp041. - DOI
-
- Burd M, Shiwakoti N, Sarvi M, Rose G. Nest architecture and traffic flow: large potential effects from small structural features. Ecol Entomol. 2010;35:464–468. doi: 10.1111/j.1365-2311.2010.01202.x. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
