Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages
- PMID: 28758133
- PMCID: PMC5516745
- DOI: 10.1155/2017/4607491
Probiotic Lactobacillus Strains Stimulate the Inflammatory Response and Activate Human Macrophages
Abstract
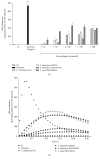
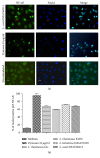
Lactobacilli have been shown to promote health functions. In this study, we analyzed the mechanism by which four different strains of probiotics affected innate immunity, such as regulation of ROS, cytokines, phagocytosis, bactericidal activity, signaling by NF-κB pp65, and TLR2 activation. The production of ROS was dependent on the concentration and species of Lactobacillus. The results obtained from the tested strains (Lactobacillus rhamnosus GG, L. rhamnosus KLSD, L. helveticus IMAU70129, and L. casei IMAU60214) showed that strains induced early proinflammatory cytokines such as IL-8,TNF-α, IL-12p70, and IL-6. However, IL-1β expression was induced only by L. helveticus and L. casei strains (after 24 h stimulation). Phagocytosis and bactericidal activity of macrophages against various pathogens, such as S. aureus, S. typhimurium, and E. coli, were increased by pretreatment with Lactobacillus. The nuclear translocation NF-κB pp65 and TLR2-dependent signaling were also increased by treatment with the probiotics. Taken together, the experiments demonstrate that probiotic strains of Lactobacillus exert early immunostimulatory effects that may be directly linked to the initial inflammation of the response of human macrophages.
Figures




Similar articles
-
Lactobacillus rhamnosus GG components, SLP, gDNA and CpG, exert protective effects on mouse macrophages upon lipopolysaccharide challenge.Lett Appl Microbiol. 2020 Feb;70(2):118-127. doi: 10.1111/lam.13255. Epub 2019 Dec 18. Lett Appl Microbiol. 2020. PMID: 31782817
-
Lactobacillus rhamnosus GR-1 enhances NF-kappaB activation in Escherichia coli-stimulated urinary bladder cells through TLR4.BMC Microbiol. 2012 Jan 22;12:15. doi: 10.1186/1471-2180-12-15. BMC Microbiol. 2012. PMID: 22264349 Free PMC article.
-
Probiotic Properties and Immunomodulatory Activity of Lactobacillus Strains Isolated from Dairy Products.Microorganisms. 2021 Apr 13;9(4):825. doi: 10.3390/microorganisms9040825. Microorganisms. 2021. PMID: 33924561 Free PMC article.
-
A comprehensive post-market review of studies on a probiotic product containing Lactobacillus helveticus R0052 and Lactobacillus rhamnosus R0011.Benef Microbes. 2011 Dec 1;2(4):319-34. doi: 10.3920/BM2011.0032. Benef Microbes. 2011. PMID: 22146691 Review.
-
The Anti-Inflammatory and Curative Exponent of Probiotics: A Comprehensive and Authentic Ingredient for the Sustained Functioning of Major Human Organs.Nutrients. 2024 Feb 16;16(4):546. doi: 10.3390/nu16040546. Nutrients. 2024. PMID: 38398870 Free PMC article. Review.
Cited by
-
Bifidobacterium breve Alleviates DSS-Induced Colitis in Mice by Maintaining the Mucosal and Epithelial Barriers and Modulating Gut Microbes.Nutrients. 2022 Sep 6;14(18):3671. doi: 10.3390/nu14183671. Nutrients. 2022. PMID: 36145047 Free PMC article.
-
Isolation, characterization, and immunomodulatory activity evaluation of probiotic strains from colostrum and canine milk.Front Vet Sci. 2023 Nov 23;10:1266064. doi: 10.3389/fvets.2023.1266064. eCollection 2023. Front Vet Sci. 2023. PMID: 38076565 Free PMC article.
-
Lactobacilli Isolated From Wild Boar (Sus scrofa) Antagonize Mycobacterium bovis Bacille Calmette-Guerin (BCG) in a Species-Dependent Manner.Front Microbiol. 2019 Jul 30;10:1663. doi: 10.3389/fmicb.2019.01663. eCollection 2019. Front Microbiol. 2019. PMID: 31417502 Free PMC article.
-
Probiotic Cell-Free Supernatants Exhibited Anti-Inflammatory and Antioxidant Activity on Human Gut Epithelial Cells and Macrophages Stimulated with LPS.Evid Based Complement Alternat Med. 2018 Jul 4;2018:1756308. doi: 10.1155/2018/1756308. eCollection 2018. Evid Based Complement Alternat Med. 2018. PMID: 30069221 Free PMC article.
-
Priming with intranasal lactobacilli prevents Pseudomonas aeruginosa acute pneumonia in mice.BMC Microbiol. 2021 Jun 28;21(1):195. doi: 10.1186/s12866-021-02254-7. BMC Microbiol. 2021. PMID: 34182930 Free PMC article.
References
-
- Reid G., Food and Agricultural Organization of the United Nation and the WHO The importance of guidelines in the development and application of probiotics. Current Pharmaceutical Design. 2005;11(1):11–16. - PubMed
-
- Delcenserie V., Martel D., Lamoureux M., Amiot J., Boutin Y., Roy D. Immunomodulatory effects of probiotics in the intestinal tract. Current Issues in Molecular Biology. 2008;10(1-2):37–54. - PubMed
-
- Vanderhoof J. A., Mitmesser S. H. Probiotics in the management of children with allergy and other disorders of intestinal inflammation. Beneficial Microbes. 2010;1(4):352–356. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

