Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation)
- PMID: 28758203
- PMCID: PMC6483410
- DOI: 10.1002/14651858.CD009377.pub3
Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation)
Abstract
Background: Haloperidol used alone is recommended to help calm situations of aggression or agitation for people with psychosis. It is widely accessible and may be the only antipsychotic medication available in limited-resource areas.
Objectives: To examine whether haloperidol alone is an effective treatment for psychosis-induced aggression or agitation, wherein clinicians are required to intervene to prevent harm to self and others.
Search methods: We searched the Cochrane Schizophrenia Group's Study-Based Register of Trials (26th May 2016). This register is compiled by systematic searches of major resources (including AMED, BIOSIS CINAHL, Embase, MEDLINE, PsycINFO, PubMed, and registries of clinical trials) and their monthly updates, handsearches, grey literature, and conference proceedings, with no language, date, document type, or publication status limitations for inclusion of records into the register.
Selection criteria: Randomised controlled trials (RCTs) involving people exhibiting aggression and/or agitation thought to be due to psychosis, allocated rapid use of haloperidol alone (by any route), compared with any other treatment. Outcomes of interest included tranquillisation or asleep by 30 minutes, repeated need for rapid tranquillisation within 24 hours, specific behaviours (threat or injury to others/self), adverse effects. We included trials meeting our selection criteria and providing useable data.
Data collection and analysis: We independently inspected all citations from searches, identified relevant abstracts, and independently extracted data from all included studies. For binary data we calculated risk ratio (RR), for continuous data we calculated mean difference (MD), and for cognitive outcomes we derived standardised mean difference (SMD) effect sizes, all with 95% confidence intervals (CI) and using a fixed-effect model. We assessed risk of bias for the included studies and used the GRADE approach to produce 'Summary of findings' tables which included our pre-specified main outcomes of interest.
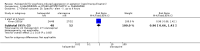
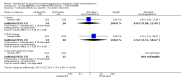
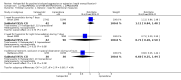
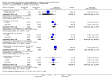
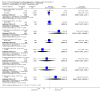
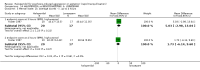
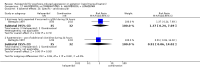
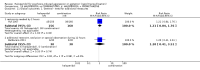
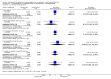
Main results: We found nine new RCTs from the 2016 update search, giving a total of 41 included studies and 24 comparisons. Few studies were undertaken in circumstances that reflect real-world practice, and, with notable exceptions, most were small and carried considerable risk of bias. Due to the large number of comparisons, we can only present a summary of main results.Compared with placebo, more people in the haloperidol group were asleep at two hours (2 RCTs, n=220, RR 0.88, 95%CI 0.82 to 0.95, very low-quality evidence) and experienced dystonia (2 RCTs, n=207, RR 7.49, 95%CI 0.93 to 60.21, very low-quality evidence).Compared with aripiprazole, people in the haloperidol group required fewer injections than those in the aripiprazole group (2 RCTs, n=473, RR 0.78, 95%CI 0.62 to 0.99, low-quality evidence). More people in the haloperidol group experienced dystonia (2 RCTs, n=477, RR 6.63, 95%CI 1.52 to 28.86, very low-quality evidence).Four trials (n=207) compared haloperidol with lorazepam with no significant differences with regard to number of participants asleep at one hour (1 RCT, n=60, RR 1.05, 95%CI 0.76 to 1.44, very low-quality of evidence) or those requiring additional injections (1 RCT, n=66, RR 1.14, 95%CI 0.91 to 1.43, very low-quality of evidence).Haloperidol's adverse effects were not offset by addition of lorazepam (e.g. dystonia 1 RCT, n=67, RR 8.25, 95%CI 0.46 to 147.45, very low-quality of evidence).Addition of promethazine was investigated in two trials (n=376). More people in the haloperidol group were not tranquil or asleep by 20 minutes (1 RCT, n=316, RR 1.60, 95%CI 1.18 to 2.16, moderate-quality evidence). Acute dystonia was too common in the haloperidol alone group for the trial to continue beyond the interim analysis (1 RCT, n=316, RR 19.48, 95%CI 1.14 to 331.92, low-quality evidence).
Authors' conclusions: Additional data from new studies does not alter previous conclusions of this review. If no other alternative exists, sole use of intramuscular haloperidol could be life-saving. Where additional drugs are available, sole use of haloperidol for extreme emergency could be considered unethical. Addition of the sedating promethazine has support from better-grade evidence from within randomised trials. Use of an alternative antipsychotic drug is only partially supported by fragmented and poor-grade evidence. Adding a benzodiazepine to haloperidol does not have strong evidence of benefit and carries risk of additional harm.After six decades of use for emergency rapid tranquillisation, this is still an area in need of good independent trials relevant to real-world practice.
Conflict of interest statement
Melanie J Powney ‐ none known.
Clive E Adams ‐ was a principle investigator on one trial included in this review (Huf 2007) .
Edoardo G Ostinelli ‐ none known.
Xue Li ‐ at the time of writing the review, Xue was employed by Systematic Review Solutions Ltd, a company that produces sytematic reviews.
Figures

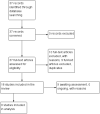
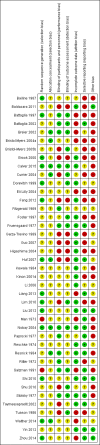































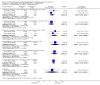




























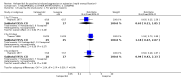



























































































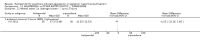













































































































Update of
-
Haloperidol for psychosis-induced aggression or agitation (rapid tranquillisation).Cochrane Database Syst Rev. 2012 Nov 14;11:CD009377. doi: 10.1002/14651858.CD009377.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2017 Jul 31;7:CD009377. doi: 10.1002/14651858.CD009377.pub3. PMID: 23152276 Updated.
References
References to studies included in this review
Bailine 1987 {published data only}
-
- Bailine SH, Lesser MS, Krubit G, Ravasz TJ, Davis RA, Kane JM. Comparison of IM haloperidol and IM chlorpromazine in the treatment of acutely psychotic patients. Psychiatric Hospital 1987;18(3):127‐9.
Baldacara 2011 {published data only}
-
- Baldacara L, Sanches M, Cordeiro DC, Jackoswski AP. Rapid tranquilization for agitated patients in emergency psychiatric rooms: A randomized trial of olanzapine, ziprasidone, haloperidol plus promethazine, haloperidol plus midazolam and haloperidol alone. Revista Brasileira de Psiquiatria 2011; Vol. 33, issue 1:30‐9. - PubMed
Battaglia 1997 {published data only}
-
- Battaglia J, Moss S, Rush J, Kang J, Mendoza R, Leedom L, et al. Haloperidol, lorazepam, or both for psychotic agitation? A multicenter, prospective, double‐blind, emergency department study. American Journal of Emergency Medicine 1997;15(4):335‐40. [MEDLINE: ] - PubMed
Battaglia 2002 {published data only}
-
- Battaglia J, David SR, Alaka K, Meehan K, Wright P. Calming versus sedative effects of IM olanzapine in agitated patients. Schizophrenia Research 2002;53(3 Suppl 1):183. - PubMed
-
- Battaglia J, Houston JP, Ahl J, Meyers AL, Kaiser CJ. A post hoc analysis of transitioning to oral treatment with olanzapine or haloperidol after 24‐hour intramuscular treatment in acutely agitated adult patients with schizophrenia. Clinical Therapeutics 2005;27(10):1612‐8. [MEDLINE: ] - PubMed
-
- Battaglia J, Lindborg SR, Alaka K, Meehan K, Wright P. Calming versus sedative effects of intramuscular olanzapine in agitated patients. American Journal of Emergency Medicine 2003;21(3):192‐8. [MEDLINE: ] - PubMed
-
- David SR, Battaglia J, Alaka K, Meehan K, Wright P. Calming versus sedative effects of IM olanzapine in agitated patients. Proceedings of the 11th Congress of the Association of European Psychiatrists; 2002 May 4‐8; Stockholm, Sweden 2002;17(Suppl 1):104s.
-
- David SR, Beasley CM Jr, Alaka K. Analysis of the QTC interval in acutely agitated patients with schizophrenia, bipolar mania, or dementia treated with intramuscular (IM) olanzapine vs. IM placebo or IM haloperidol. European Neuropsychopharmacology 2001;11(3):276.
Breier 2002 {published data only}
-
- Breier A, Meehan K, Birkett M, David S, Ferchland I, Sutton V, et al. A double‐blind, placebo‐controlled dose‐response comparison of intramuscular olanzapine and haloperidol in the treatment of acute agitation in schizophrenia. Archives of General Psychiatry 2002;59(5):441‐8. - PubMed
-
- Breier A, Wright P, Birkett M, David S, Brook S, Meehan K. A double‐blind dose response study comparing intramuscular (IM) olanzapine, haloperidol, and placebo in acutely agitated schizophrenic patients. Biological Psychiatry 2001;49(8):121S.
-
- Breier A, Wright P, Birkett M, Meehan K, David S, Brook S. A double‐blind dose response study comparing intramuscular olanzapine, haloperidol and placebo in acutely agitated schizophrenic patients. Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000 Dec 10‐14; San Juan, Puerto Rico. 2000.
-
- Breier AF, Wright P, Birkett M, Meehan K, David SR, Brook S. Intramuscular olanzapine: dose‐related improvement in acutely agitated patients with schizophrenia. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans, Louisiana, USA. Marathon Multimedia, 2001.
-
- Smith RC. 10 mg intramuscular olanzapine reduces acute agitation in schizophrenia more effectively than lower doses [Commentary]. Evidence‐Based Mental Health 2003;6(1):27. [MEDLINE: ] - PubMed
Bristol Myers 2004a {published data only}
-
- Andrezina R, Josiassen RC, Marcus RN, Oren DA, Manos G, Stock E, et al. Intramuscular aripiprazole for the treatment of acute agitation in patients with schizophrenia or schizoaffective disorder: a double‐blind, placebo‐controlled comparison with intramuscular haloperidol. Psychopharmacology 2006;188(3):281‐92. [MEDLINE: ] - PubMed
-
- Andrezina R, Marcus RN, Oren DA, Manos G, Stock E, Carson WH, et al. Intramuscular aripiprazole or haloperidol and transition to oral therapy in patients with agitation associated with schizophrenia: sub‐analysis of a double‐blind study. Current Medical Research and Opinion 2006;22(11):2209‐19. [MEDLINE: ] - PubMed
-
- Bristol‐Myers S. A randomized, double‐blind comparison of the efficacy and safety of aripiprazole intramuscular formula, haloperidol, or placebo in the treatment of acutely agitated patients with a diagnosis of schizophrenia or schizoaffective disorder. http://www.clinicalstudyresults.org/ 2004.
-
- Citrome LL, Vester‐Blokland E, Archibald D, McQuade R, Kostic D, Pikalov A, et al. Benefits of a second dose of intramuscular (IM) aripiprazole to control agitation in patients with schizophrenia or bipolar I disorder. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25, Toronto, Canada. 2006.
-
- Currier GW, Crandall D, Archibald D, Nyilas M, Kostic D, Pikalov A, et al. Intramuscular (IM) aripiprazole controls agitation in patients with schizophrenia or bipolar disorder without excessive sedation. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
Bristol‐Myers 2005b {published data only}
-
- Bristol‐Myers S. Randomized, double‐blind, dose‐ranging study of intramuscular aripiprazole in the treatment of acute agitation in patients with a diagnosis of schizophrenia, schizoaffective, or schizophreniform disorder. http://www.clinicalstudyresults.org/ 2005.
-
- Currier GW, Crandall D, Archibald D, Nyilas M, Kostic D, Pikalov A, et al. Intramuscular (IM) aripiprazole controls agitation in patients with schizophrenia or bipolar disorder without excessive sedation. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
-
- Gismondi R, Daniel D, Stock E, Wilber R, Marcus R, Carson W, et al. Intramuscular aripiprazole treatment for acute agitation in patients with psychosis. European Neuropsychopharmacology 2004;14(Suppl 3):S261.
-
- Kungel M, Czaniera R, Ebrecht M, Werner C, Oren D, McQuade R, et al. Intramuscular aripiprazole for the treatment of acute agitation accompanied with schizophrenia: Subanalysis of a double‐blind controlled study to find the dosage. Proceedings of the 25th Symposium of the Arbeitsgemeinschaft Neuropsychopharmakologie und Pharmakopsychiatrie; 2007 Oct 3‐6; Munich, Germany. 2007.
-
- Kungel M, Daniel D, Stock E, Wilber R, Marcus R, Carson W, et al. Efficacy and safety of intramuscular aripiprazole in acutely agitated patients with psychosis. Proceedings of the Thematic Conference of the World Psychiatric Association on "Treatments in Psychiatry: An Update"; 2004 Nov 10‐13; Florence, Italy. 2004.
Brook 2000 {published data only}
-
- Brook S, Krams M, Gunn K. Intramuscular (IM) ziprasidone vs. IM haloperidol in patients with acute, non‐organic psychosis. Proceedings of the 9th Congress of the Association of European Psychiatrists; 1998 Sep 20‐24; Copenhagen, Denmark. 1998.
-
- Brook S, Krams M, Gunn KP. Intramuscular ziprasidone versus intramuscular haloperidol. Proceedings of the 21st Collegium Internationale Neuro‐Psychopharmacologicum Congress; 1998 Jul 12‐16; Glasgow, UK 1998.
-
- Brook S, Krams M, Gunn KP, and the Ziprasidone IM Study Group. The efficacy and tolerability of intramuscular (IM) ziprasidone versus IM haloperidol in patients with acute non‐organic psychosis. Proceedings of the 151st Annual Meeting of the American Psychiatric Association; 1998 May 30 ‐ Jun 4; Toronto, Ontario, Canada. 1998.
-
- Brook S, Lucey JV, Gunn KP. Intramuscular ziprasidone compared with intramuscular haloperidol in the treatment of acute psychosis. Journal of Clinical Psychological Medicine [临床精神医学杂志] 2000;61(12):933‐41. - PubMed
-
- Daniel DG, Brook S, Reeves KR. An overview of the efficacy and safety of rapid acting intramuscular ziprasidone. European Neuropsychopharmacology 2000;10(Suppl 3):S239.
Calver 2015 {published data only}
-
- Calver L, Drinkwater V, Gupta R, Page CB, Isbister GK. Droperidol v. haloperidol for sedation of aggressive behaviour in acute mental health: Randomized controlled trial. British Journal of Psychiatry 2015; Vol. 206, issue 3:223‐8. - PubMed
Currier 2004 {published data only}
-
- Currier GW, Chou JC, Feifel D, Bossie CA, Turkoz I, Mahmoud R, et al. Acute treatment of psychotic agitation: a randomized comparison of oral treatment with risperidone and lorazepam versus intramuscular treatment with haloperidol and lorazepam. Journal of Clinical Psychiatry 2004;65(3):386‐94. [MEDLINE: ] - PubMed
Dorevitch 1999 {published data only}
-
- Dorevitch A, Katz N, Zemishlany Z, Aizenberg D, Weizman A. Intramuscular flunitrazepam versus intramuscular haloperidol in the emergency treatment of aggressive psychotic behavior. American Journal of Psychiatry 1999;156(1):142‐4. - PubMed
Eli Lilly 2004 {published data only}
-
- Chan HY, Ree SC, Su LW, Chen JJ, Chou SY, Chen CK, et al. A double‐blind, randomized comparison study of efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia and acute agitated behavior. Journal of Clinical Psychopharmacology 2014; Vol. 34, issue 3:355‐8. - PubMed
-
- Eli Lilly. Comparison of intramuscular olanzapine and intramuscular haloperidol in patients with schizophrenia. Eli Lilly and Company Clinical Trial Registry 2004.
-
- NCT00485901. A double‐blind randomized comparison of the efficacy and safety of intramuscular olanzapine and intramuscular haloperidol in acutely agitated patients with schizophrenia. http://www.clinicaltrials.gov 2007.
Fang 2012 {published data only}
-
- Fang M, Chen H, Li LH, Wu R, Li Y, Liu L, et al. Comparison of risperidone oral solution and intramuscular haloperidol with the latter shifting to oral therapy for the treatment of acute agitation in patients with schizophrenia. International Clinical Psychopharmacology 2012; Vol. 27, issue 2:107‐13. - PubMed
Fitzgerald 1969 {published data only}
-
- Fitzgerald CH. A double‐blind comparison of haloperidol with perphenazine in acute psychiatric episodes. Current Therapeutic Research 1969;11(8):515‐9. - PubMed
Foster 1997 {published data only}
-
- Foster S, Kessel J, Berman ME, Simpson GM. Efficacy of lorazepam and haloperidol for rapid tranquilization in a psychiatric emergency room setting. International Clinical Psychopharmacology 1997;12(3):175‐9. [MEDLINE: ] - PubMed
Fruensgaard 1977 {published data only}
-
- Fruensgaard K, Korsgaard S, Jorgensen H, Jensen K. Loxapine versus haloperidol parenterally in acute psychosis with agitation. A double‐blind study. Acta Psychiatrica Scandinavica 1977;56(4):256‐64. [MEDLINE: ] - PubMed
Garza‐Trevino 1989 {published data only}
-
- Garza‐Trevino ES, Hollister LE, Overall JE, Alexander WF. Efficacy of combinations of intramuscular antipsychotics and sedative‐hypnotics for control of psychotic agitation. American Journal of Psychiatry 1989;146(12):1598‐601. - PubMed
Guo 2007 {published data only}
-
- Guo C‐R. Study of quetiapine combined with magnesium valproate release tablets in treatment of schizophrenia with symptoms of elation and agitation [奎硫平合并丙戊酸镁缓释片治疗精神分裂症兴奋激越研究]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2007;17(3):183‐4.
Higashima 2004 {published data only}
-
- Higashima M, Takeda T, Nagasawa T, Hirao N, Oka T, Nakamura M, et al. Combined therapy with low‐potency neuroleptic levomepromazine as an adjunct to haloperidol for agitated patients with acute exacerbation of schizophrenia. European Psychiatry 2004;19(6):380‐1. [MEDLINE: ] - PubMed
Huf 2007 {published data only}
-
- Barbui C. Intramuscular haloperidol plus promethazine is more effective and safer than haloperidol alone for rapid tranquillisation of agitated mentally ill patients. Evidence‐Based Mental Health 2008;11(3):86‐7. - PubMed
-
- Huf G. Haloperidol plus promethazine versus haloperidol for psychosis induced aggression. Data on file 2004.
-
- Huf G, Coutinho ESF, Adams CE. The pharmacological management of agitated patients in emergency psychiatric hospitals in Rio de Janeiro, Brazil: the results of two pragmatic randomized clinical trials. Proceedings of the 5th European Congress on Violence in Clinical Psychiatry; 2007 Oct 25‐27; Amsterdam, The Netherlands. 2007.
-
- ISRCTN83261243. TREC2 ‐ Rapid tranquillisation for agitated patients in emergency psychiatric rooms in Rio de Janeiro. A randomised trial of intramuscular haloperidol versus intramuscular haloperidol + promethazine. http://www.controlled‐trials.com 2005.
Kewala 1984 {published data only}
-
- Kewala S, Ban TA, Berney SA, Wilson WH. Rapid tranquilization: A comparative study of thiothixene and haloperidol. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 1984;8:77‐83. - PubMed
Kinon 2001d {published data only}
-
- Kinon B, Rotelli MD, Gilmore JA. Efficacy of olanzapine and adjunctive lorazepam to control agitation in schizophrenia. International Journal of Neuropsychopharmacology 2002;5(Suppl 1):S128.
-
- Kinon BJ, Ahl J, Rotelli MD, McMullen E. Efficacy of accelerated dose titration of olanzapine with adjunctive lorazepam to treat acute agitation in schizophrenia. American Journal of Emergency Medicine 2004;22(3):181‐6. [MEDLINE: ] - PubMed
-
- Kinon BJ, Wang L, Rotelli MD, Gilmore JA. The efficacy of olanzapine plus adjunctive lorazepam to control acute agitation in schizophrenia. European Neuropsychopharmacology 2001;11(3):278.
Li 2006 {published data only}
-
- Li L‐H, Zhao J‐P, Xu X‐F. A comparative study of intramuscular ziprasidone and haloperidol in treating acute agitation in schizophrenia [国产齐拉西酮与氟哌啶醇注射液治疗精神分裂症急性激越症状的对照研究]. Chinese Journal of Psychiatry 2006;39(4):216‐9.
Liang 2013 {published data only}
-
- Liang Y, Su Y, Huang J, Tang M, Li K, Yang F, et al. Effect of quetiapine and haloperidol on QTc interval in treating schizophrenic patients with excitement and agitation [喹硫平和氟哌啶醇治疗精神分裂症急性期兴奋激越对QTc间期的影响]. Chinese New Drugs Journal [Zhongguo Xin Yao Za Zhi; 中国新药杂志] 2013; Vol. 22, issue 16:1886‐9+1894.
-
- Liang Y, Su Y, Huang J, Tang M, Li K, Yang P, et al. A randomized controlled study of effect and safety of quetiapine and haloperidol in treatment of schizophrenic patients with excitement and agitation [喹硫平和氟哌啶醇治疗精神分裂症急性期兴奋激越的多中心随机对照研究]. Chinese Mental Health Journal [Zhongguo Xin Li Wei Sheng Za Zhi; 中国心理卫生杂志] 2013; Vol. 27, issue 4:262‐7.
Lim 2010 {published data only}
-
- Kim JJ, Lim HK, Lee CU, Lee C, Pae CU, Paik IH. Risperidone orodispersible tablet and intramuscular haloperidol in treatment of acute psychotic agitation. European Neuropsychopharmacology 2007;17(Suppl 4):S467. - PubMed
-
- Lim HK, Kim JJ, Pae CU, Lee CU, Lee C, Paik IH. Comparison of risperidone orodispersible tablet and intramuscular haloperidol in the treatment of acute psychotic agitation: A randomized open, prospective study. Neuropsychobiology 2010;62(2):81‐6. [ISI: 000280037400001] - PubMed
Liu 2012 {published data only}
-
- 刘锟, 刘云, 宁南义, 孙建中, 孙亚军, 孙同勋, et al. Consolidation of short‐term risperidone intramuscular haloperidol in the treatment of acute agitation in schizophrenia randomized controlled study of the efficacy of behavior [Google Translate] [利培酮合并短期肌注氟哌啶醇治疗精神分裂症急性期激越行为疗效的随机对照研究]. 中国医药导报 2012, issue 35:104‐6.
Man 1973 {published data only}
-
- Man‐Pang L, Chen CH. Rapid tranquilization of acutely psychotic patients with intramuscular haloperidol and chlorpromazine. Psychosomatics 1973;14(1):59‐63. [MEDLINE: ] - PubMed
Nobay 2004 {published data only}
-
- Nobay F, Simon BC, Levitt MA, Dresden GM. A prospective, double‐blind, randomized trial of midazolam versus haloperidol versus lorazepam in the chemical restraint of violent and severely agitated patients. Academic Emergency Medicine 2004;11(7):744‐9. [MEDLINE: ] - PubMed
Paprocki 1977 {published data only}
-
- Paprocki J, Versiani M. A double‐blind comparison between loxapine and haloperidol by parenteral route in acute schizophrenia. Current Therapeutic Research 1977;21(1):80‐100. - PubMed
Reschke 1974 {published data only}
-
- Reshke RW. Parenteral haloperidol for rapid control of severe, disruptive symptoms of acute schizophrenia. Diseases of the Nervous System 1974;35:112‐5. - PubMed
Resnick 1984 {published data only}
-
- Resnick M, Burton BT. Droperidol vs. haloperidol in the initial management of acutely agitated patients. Journal of Clinical Psychological Medicine [临床精神医学杂志] 1984;45(7):298‐9. - PubMed
Ritter 1972 {published data only}
-
- Ritter RM, Davidson DE, Robinson TA. Comparison of injectable haloperidol and chlorpromazine. American Journal of Psychiatry 1972;129(1):78‐81. [MEDLINE: ] - PubMed
Salzman 1991 {published data only}
-
- Salzman C, Solomon D, Miyawaki E, Glassman R, Rood L. Parenteral lorazepam versus parenteral haloperidol for the control of psychotic disruptive behavior. Journal of Clinical Psychiatry 1991;52(4):177‐80. [MEDLINE: ] - PubMed
Shi 2010 {published data only}
-
- Shi Y, Dai T, Yi P. olanzapine combined magnesium valproate sustained‐release tablets in the treatment of schizophrenia, acute agitation excited [Google Translate] [奥氮平合并丙戊酸镁缓释片治疗精神分裂症急性期兴奋激越研究]. Modern Practical Medicine 2010; Vol. 22, issue 4:398‐9.
Shu 2010 {published data only}
-
- NCT00723606. A randomized, open‐label, multi‐center study to evaluate the efficacy and safety of intramuscular ziprasidone in patients with agitation. http://www.clinicaltrials.gov 2008.
-
- Pfizer. A randomized, open label, rater blind, flexible dose multi‐center study comparing the efficacy and safety of intramuscular ziprasidone with haloperidol for three days in patients with agitation of schizophrenia. http://www.clinicalstudyresults.org/documents/company‐study_10505_0.pdf (accessed 13 June 2011).
-
- Shu L, Zhang H, Wang G, Zhao J, Xie S, Xu X, et al. Intramuscular ziprasidone has improved tolerability over haloperidol and comparable efficacy for control of agitation in schizophrenia in Chinese patients. Schizophrenia Research 2010;117(2‐3):260.
-
- Zhang H, Wang G, Zhao J, Xie S, Xu X, Shi J, et al. Intramuscular ziprasidone versus haloperidol for managing agitation in Chinese patients with schizophrenia. Journal of Clinical Psychopharmacology 2013; Vol. 33, issue 2:178‐85. - PubMed
Stotsky 1977 {published data only}
-
- Stotsky BA. Relative efficacy of parenteral haloperidol and thiothixene for the emergency treatment of acutely excited and agitated patients. Diseases of the Nervous System 1977;38(12):967‐73. [MEDLINE: ] - PubMed
Taymeeyapradit 2002 {published data only}
-
- Taymeeyapradit U, Kuasirikul S. Comparative study of the effectiveness of zuclopenthixol acetate and haloperidol in acutely disturbed psychotic patients. Journal of the Medical Association of Thailand 2002;85(12):1301‐8. [MEDLINE: ] - PubMed
Tuason 1986 {published data only}
-
- Tuason VB. A comparison of parenteral loxapine and haloperidol in hostile and aggressive acutely schizophrenic patients. Journal of Clinical Psychiatry 1986;47(3):126‐9. [MEDLINE: ] - PubMed
Walther 2014 {published data only}
-
- Walther S, Moggi F, Horn H, Moskvitin K, Abderhalden C, Maier N, et al. Rapid tranquilization of severely agitated patients with schizophrenia spectrum disorders: a naturalistic, rater‐blinded, randomized, controlled study with oral haloperidol, risperidone, and olanzapine. Journal of Clinical Psychopharmacology 2014; Vol. 34, issue 1:124‐8. - PubMed
-
- Walther S, Moggi F, Horn H, Moskvitin K, Maier N, Abderhalden C, et al. Rapid tranquilization of severely agitated patients with schizophrenia spectrum disorders: A naturalistic, rater‐blinded, randomized controlled study with oral haloperidol, risperidone, and olanzapine. PharmacoPsychiatry 2013; Vol. 46, issue 6:A77. - PubMed
Yin 2012 {published data only}
-
- 尹析凡, 王刚. 齐拉西酮注射液治疗精神分裂症激越症状的研究. 中国当代医药 2012; Vol. 30:9‐11.
Zhou 2014 {published data only}
-
- 周升宝, 孙晓丹, 庞琦, 李志梅, 赵继舒, 张峰. 齐拉西酮联合氯硝西泮治疗精神分裂症急性期兴奋激越症状对照研究. 临床心身疾病杂志 2014; Vol. 20, issue 2:17‐20.
References to studies excluded from this review
Addington 1996 {published data only}
-
- Addington DE, Arvanitis LA. 'Seroquel' (quetiapine): an atypical antipsychotic ‐ results from a multiple fixed dose, placebo‐controlled study. Proceedings of the 4th International Conference on Schizophrenia ‐ 1996: Breaking down the Barriers; 1996 Oct 6‐9; Vancouver, Canada. 1996.
-
- Arvanitis LA, Sweitzer DE, Goldstein JM, Yeung PP. Serequel reduces aggression in patients with acute exacerbation of schizophrenia. Proceedings of the 11th European College of Neuropsychopharmacology Congress; 1998 Oct 31 ‐ Nov 4; Paris, France. 1998.
-
- Borison RL, Arvanitist LA, Miller BG. A comparison of five fixed doses 'seroquel' (ICI 204,636) with haloperidol and placebo in patients with schizophrenia. Schizophrenia Research 1996;18(2‐3):132.
-
- Cantillon M. Quetiapine fumarate reduces aggression. Proceedings of the 11th Annual Meeting of the American Association for Geriatric Psychiatry; 1998 Mar 8‐11; San Diego, California, USA. 1998.
-
- Cantillon M, Arvanitis LA, Miller BG, Kowalcyk. 'Seroquel' (quetiapine fumarate) reduces hositility and aggression in patients with acute schizophrenia. Proceedings of the 36th Annual Meeting of the American College of Neuropsychopharmacology; 1997 Dec 8‐12; Waikoloa, Hawaii, USA. 1997.
Alexander 2004 {published data only}
-
- Alexander J. Lorazepam versus a combination of haloperidol and promethazine in the acute management of agitation and aggression ‐ a randomized controlled trial [thesis]. Guindy, Chennai: Tamilnadu Dr. MGR Medical University, 2003.
-
- Alexander J, John T, Tharyan P, Adams CE. TREC‐India. A second arm of TREC. Schizophrenia Research 2002;53(3 Suppl 1):236.
-
- Alexander J, Tharyan P, Adams C, John T, Mol C, Philip J. Rapid tranquillisation of violent or agitated patients in a psychiatric emergency setting. Pragmatic randomised trial of intramuscular lorazepam v haloperidol plus promethazine. British Journal of Psychiatry 2004;185:63‐9. - PubMed
-
- NCT00455234. Rapid tranquilization of violent or agitated people in psychiatric emergency settings‐ a pragmatic randomized controlled trial of intramuscular olanzapine vs intramuscular haloperidol + promethazine. http://www.clinicaltrials.gov 2007.
-
- Raveendran NS. Rapid tranquillization of acutely agitated patients: intramuscular olanzapine vs haloperidol + promethazine ‐ pragmatic randomized control trial. Proceedings of the 5th European Congress on Violence in Clinical Psychiatry; 2007 Oct 25‐27; Amsterdam, The Netherlands. 2007.
Allen 2007 {published data only}
-
- Allen M, Talbott S, Eudicone J, McQuade R, Pikalov A. Similar rates of agitation, anxiety and insomnia in sedating and non‐sedating antipsychotics: evaluating clinical trial results with aripiprazole, haloperidol and olanzapine. Schizophrenia Bulletin 2007;33(2):418.
Asadollahi 2015 {published data only}
-
- Asadollahi S, Heidari K, Hatamabadi H, Vafaee R, Yunesian S, Azadbakht A, et al. Efficacy and safety of valproic acid versus haloperidol in patients with acute agitation: results of a randomized, double‐blind, parallel‐group trial. International Clinical Psychopharmacology 2015; Vol. 30, issue 3:142‐50. - PubMed
Beasley 1998 {published data only}
-
- Beasley CM, Sayler ME, Keisler GM, Potvin JH, Sanger TM, Tollefson GD. The influence of pharmacotherapy on self‐directed and externally‐directed aggression in schizophrenia. Schizophrenia Research 1998;29(1‐2):28.
Bieniek 1998 {published data only}
-
- Bieniek SA, Ownby RL, Penalver A, Dominguez RA. A double‐blind study of lorazepam versus the combination of haloperidol and lorazepam in managing agitation. Pharmacotherapy 1998;18(1):57‐62. - PubMed
Brook 1998 {published data only}
-
- Brook S, Berk M, Selemani S, Kolloori J, Nzo I. A randomized controlled double blind study of zuclopenthixol acetate compared to haloperidol in acute psychosis. Human Psychopharmacology 1998;13(1):17‐20.
Campbell 1982 {published data only}
-
- Campbell M, Cohen IL, Small AM. Drugs in aggressive behavior. Journal of the American Academy of Child Psychiatry 1982;21(2):107‐17. [MEDLINE: ] - PubMed
Chen 2004 {published data only}
-
- Chen M‐F, Liu D‐X, Lin J‐Z. The comparison study on curing agitation by haloperidol and chlorpromazine venoclysis [氟哌啶醇与氯丙嗪静滴治疗急性激越的对照研究]. Medical Journal of Chinese Civil Administration 2004;16(10):599.
Chen 2008 {published data only}
-
- Chen J, Yang X, Song A, Gu X, Ma X. Intramuscular ziprasidone versus haloperidol in the treatment of acute agitation in schizophrenia [齐拉西酮注射液与氟哌啶醇注射液治疗精神分裂症急性激越症状的对比研究]. Shanghai Archives of Psychiatry [上海精神医学] [上海精神醫學] 2008;20(6):363‐6.
Chouinard 1991 {published data only}
-
- Chouinard G, Annable L, Turnier L, Holobow N, Szkrumelak N. A double‐blind randomized clinical trial of rapid tranquilization with IM clonazepam and IM haloperidol in agitated psychotic patients with manic symptoms. Canadian Journal of Psychiatry [Revue Canadienne de Psychiatrie] 1993;38(Suppl 4):S114‐21. [MEDLINE: ] - PubMed
-
- Chouinard G, Safadi G, Beauclair L. The management of acutely schizophrenic patients newly admitted from the emergency room: A double‐blind clinical trial comparing zuclopenthixol acetate and liquid haloperidol. Proceedings of the 5th World Congress of Biological Psychiatry; 1991 Jun 9‐14; Florence, Italy. Amsterdam, Netherlands: Excerpta Medica, 1991:35‐43.
Citrome 2001 {published data only}
-
- Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer J‐P, McEvoy J, et al. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatric Services 2001;52(11):1510‐4. [MEDLINE: ] - PubMed
-
- Citrome LL, Volavka J, Czobor P, Nolan K, Lieberman JA, Lindenmayer J‐P, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA. 2003.
-
- Citrome LL, Volavka J, Czobor P, Sheitman BB, Lindenmayer J‐P, McEvoy JP, et al. Atypical antipsychotics and hostility in schizophrenia: A double‐blind study. Proceedings of the 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans, Louisiana, USA. 2001.
-
- Citrome LL, Volavka J, Czobor P, Sheitman BB, Lindenmayer J‐P, McEvoy JP, et al. Atypical antipsychotics and hostility in schizophrenia: A double‐blind study. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, Pennsylvania, USA. 2002.
Colonna 1998 {published data only}
-
- Colonna L, Martin P, Gerard D. Long‐term study of the effectiveness and tolerance of amisulpride versus haloperidol [Etude à long terme de L'efficacité et de la tolérance à l'amisulpride versus haloperidol]. Proceedings of the 96th Congres de Psychiatrie et de Neurologie de Langue Francaise; 1998 May 10‐15; Saint Paul, France. 1998.
Conde 2011 {published data only}
-
- Conde BL, Sobreveg EE, Sionzon MP. A randomized trial of oral risperidone versus intramuscular haloperidol in the emergency treatment of acute psychotic agitation. ASEAN Journal of Psychiatry 2011; Vol. 12, issue 1:29‐36.
Crandall 2005 {published data only}
-
- Crandall D, Pikalov A, Kostic D, Kaplita S, Berman R, McQuade R, et al. Is sedation needed for effective reduction of acute schizophrenia symptoms?. Proceedings of the 158th Annual Meeting of the American Psychiatric Association; 2005 May 21‐26; Atlanta, Georgia, USA. 2005.
Currier 2000 {published data only}
-
- Currier GW, Simpson GA. Risperidone oral solution versus intramuscular haloperidol for control of psychotic agitation. European Neuropsychopharmacology 2000;10(Suppl 3):S296.
Daniel 2000 {published data only}
-
- Daniel DG, Brook S, Reeves KR. An overview of the efficacy and safety of rapid acting intramuscular ziprasidone. European Neuropsychopharmacology 2000;10(Suppl 3):S289.
Daniel 2003 {published data only}
-
- Daniel DG, Brook S, Benattia I. Efficacy of intramuscular ziprasidone without adjunctive benzodiazepines. European Neuropsychopharmacology 2003;13(4):S299.
Daniel 2004 {published data only}
-
- Daniel DG, Zimbroff DL, Swift RH, Harrigan EP. The tolerability of intramuscular ziprasidone and haloperidol treatment and the transition to oral therapy. International Clinical Psychopharmacology 2004;19(1):9‐15. - PubMed
-
- Lucey JV, Brook S, Daniel DG, Reeves KR, Harrigan EP. Intramuscular (IM) ziprasidone: a novel treatment for the short term management of agitated psychotic patients. Schizophrenia Research 2000;41(1):208.
-
- Modell S, Daniel D, Stock E, Wilber R, Marcus R, Carson W, et al. Intramuscular aripiprazole treatment for acute agitation in patients with psychosis. Proceedings of the 24th Collegium Internationale Neuro‐Psychopharmacologicum Congress; 2004 June 20–24; Paris, France 2004.
-
- Swift RH, Harrigan EP, Cappelleri JC, Kramer D, Chandler LP. Validation of the behavioural activity rating scale (BARS): a novel measure of activity in agitated patients. Journal of Psychiatric Research 2002;36(2):87‐95. [MEDLINE: ] - PubMed
-
- Swift RH, Harrigan EP, Kammen DP. A comparison of intramuscular (IM) ziprasidone with IM haloperidol. Proceedings of the 9th Congress of the Association of European Psychiatrists; 1998 Sep 20‐24; Copenhagen, Denmark. 1998.
Fan 2012 {published data only}
-
- 樊凌姿, 曹立新. 氯普噻吨注射液与氟哌啶醇注射液治疗精神分裂症兴奋激越对照研究. 中外健康文摘 2012; Vol. 40:31‐2.
Faretra 1970 {published data only}
-
- Faretra G, Dooher L, Dowling J. Comparison of haloperidol and fluphenazine in disturbed children. American Journal of Psychiatry 1970;126:1670‐3. [MEDLINE: ] - PubMed
Freeman 2009 {published data only}
-
- Freeman DJ, DiPaula BA, Love RC. Intramuscular haloperidol versus intramuscular olanzapine for treatment of acute agitation: A cost‐minimization study. Pharmacotherapy 2009;29(8):930‐6. - PubMed
Goldstein 1966 {published data only}
-
- Goldstein BJ, Clyde DJ. Haloperidol in controlling the symptoms of acute psychoses. Part II: A double blind evaluation of haloperidol and trifluoperazine. Current Therapeutic Research 1966;8:236‐40. - PubMed
Harvey 2004 {published data only}
-
- Harvey AT, Flockhart D, Gorski JC, Greenblatt DJ, Burke M, Werder S, et al. Intramuscular haloperidol or lorazepam and QT intervals in schizophrenia. Journal of Clinical Pharmacology 2004;44(10):1173‐84. [MEDLINE: ] - PubMed
Huang 2004 {published data only}
-
- Huang W‐S, Di X‐L, Yang F‐D. A comparative study of the effect of modern electroconvulsive therapy on the agitated behavior of psychotic patients. Journal of Clinical Psychological Medicine [临床精神医学杂志] 2004;14(5):276‐7.
Jiang 2009 {published data only}
-
- 蒋合萍, 罗毅平. Olanzapine and haloperidol in the treatment of schizophrenia comparative study of excited agitation [Google Translate] [奥氮平与氟哌啶醇治疗精神分裂症兴奋激越症状的对照研究]. Sichuan Mental Health [四川精神卫生] 2009; Vol. 22, issue 1:43‐4.
Jones 2001 {published data only}
-
- Jones B, Taylor CC, Meehan K. The efficacy of a rapid‐acting intramuscular formulation of olanzapine for positive symptoms. Journal of Clinical Psychological Medicine [临床精神医学杂志] 2001;62(2):22‐4. [MEDLINE: ] - PubMed
Kane 2000 {published data only}
-
- Kane J, Ingenito G, Ali M. Efficacy of aripiprazole in psychotic disorders: comparison with haloperidol and placebo. Schizophrenia Research 2000;41(1):39.
Kinon 2001a {published data only}
-
- Eli Lilly. Beasley 1996a ‐ olz vs placebo vs hpl (N America) (as supplied 2004). Data on file 2001.
-
- Eli Lilly. Beasley 1997 ‐ olz vs hpl (international) (as supplied 2004). Data on file 2001.
-
- Eli Lilly. Tollefson 1997 ‐ olz vs hpl (as supplied 2004). Data on file 2001.
-
- Kinon BJ, Basson MS, Tollefson GD. Gender‐specific prolactin response to treatment with olanzapine versus haloperidol in schizophrenia. Proceedings of the 9th Biennial Winter Workshop on Schizophrenia; 1998 Feb 7‐13; Davos, Switzerland 1998.
-
- Kinon BJ, Milton DR, Hill AL, Williamson DJ. Effective resolution of acute presentation of behavioral agitation and positive psychotic symptoms in schizophrenia with olanzapine. Journal of Psychopharmacology 2000;14(3 Suppl):A60.
Kinon 2003 {published data only}
-
- Kinon BJ, Stauffer VL, McGuire HC, Kaiser CJ, Dickson RA, Kennedy JS. The effects of antipsychotic drug treatment on prolactin concentrations in elderly patients. Journal of the American Medical Directors Association 2003;4(4):189‐94. [PUBMED: 12837139] - PubMed
-
- Kinon BJ, Wang L, Stauffer VL. A comparison of prolactin concentrations in the elderly during treatment with olanzapine, haloperidol, and risperidone. Proceedings of the 14th Annual Meeting of the American Association for Geriatric Psychiatry; 2001 Feb 23‐26; San Francisco, California, USA. 2001.
Krakowski 2006 {published data only}
-
- Krakowski M, Czobor P. Cholesterol and cognition in schizophrenia: A double‐blind study of patients randomized to clozapine, olanzapine and haloperidol. Schizophrenia Research 2011; Vol. 130, issue 1‐3:27‐33. - PubMed
-
- Krakowski M, Czobor P, Citrome L. Weight gain, metabolic parameters, and the impact of race in aggressive inpatients randomized to double‐blind clozapine, olanzapine or haloperidol. Schizophrenia Research 2009;110(1‐3):95‐102. [MEDLINE: ] - PubMed
-
- Krakowski MI. Clozapine and olanzapine in violent schizophrenics. CRISP database (https://www‐commons.cit.nih.gov/crisp/index.html) (accessed 19th February 2001.
-
- Krakowski MI, Czobor P. A prospective longitudinal study of cholesterol and aggression in patients randomized to clozapine, olanzapine, and haloperidol. Journal of Clinical Psychopharmacology 2010;30(2):198‐200. - PubMed
Krakowski 2008 {published data only}
-
- Krakowski M, Czobor P. A prospective longitudinal study of cholesterol and aggression in patients randomized to clozaplne, olanzapine and haloperidol. Proceedings of the 15th Biennial Winter Workshop in Psychoses; 2009 Nov 15‐18; Barcelona, Spain. 2009. - PubMed
-
- Krakowski MI, Czobor P, Nolan KA. Atypical antipsychotics, neurocognitive deficits, and aggression in schizophrenic patients. Journal of Clinical Psychopharmacology 2008;28(5):485‐93. - PubMed
-
- Nolan KA, Volavka J, Czobor P, Sheitman B, Lindenmayer J‐P, Citrome LL, e al. Aggression and psychopathology in treatment‐resistant inpatients with schizophrenia and schizoaffective disorder. Journal of Psychiatric Research 2005;39(1):109‐15. [MEDLINE: ] - PubMed
Lewis 2006 {published data only}
-
- Lewis CF. Haloperidol versus risperidone in the treatment of aggressive psychotic male inmates. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
-
- NCT00203775. Efficacy of risperidone versus haloperidol in the treatment of aggression and hostility in psychotic inmates. http://www.clinicaltrials.gov 2005.
Li 2005 {published data only}
-
- Li GL. Tiapride intramuscular haloperidol in the treatment of acute schizophrenia excited state control study [硫必利肌注与氟哌啶醇治疗精神分裂症急性期兴奋状态的对照研究]. Nervous Diseases and Mental Health [神经疾病与精神卫生] 2005; Vol. 5, issue 6:456‐7.
Li 2007 {published data only}
-
- Li C, Zhu S, Wang H, Chen H, Yu Y, Liu D, et al. Safety and efficacy of clonazepam, haloperidol and haloperidol combined with clonazepam in the in the treatment of schizophrenia with excitement and agitation [氯硝西泮、氟哌啶醇及其联合治疗对精神分裂症激越症状疗效的比较]. Shanghai Archives of Psychiatry [上海精神医学] [上海精神醫學] 2007;19(3):150‐2.
Liang 2003 {published data only}
-
- 梁芝国, 罗维肖. Clonazepam and haloperidol on patients with acute agitation control study [氯硝西泮和氟哌啶醇联用治疗急性激越的对照研究]. Medical Journal of Chinese Civil Administration 2003; Vol. 15, issue 5:293‐4.
Liu 2012b {published data only}
-
- 刘庆梅. 无抽搐电休克治疗精神分裂症伴激越行为疗效观察及护理干预. 临床心身疾病杂志 2012; Vol. 18, issue 5:437‐9.
Mandel 2008 {published data only}
-
- Mandel F, Allen MH, Lombardo I. Early response to intramuscular ziprasidone as a predictor of end point response to oral ziprasidone. Proceedings of the 161st Annual Meeting of the American Psychiatric Association; 2008 May 3‐8; Washington DC, USA. 2008.
Mantua 2006 {published data only}
-
- Mantua V, Travis MJ, Atakan Z, Isaac MB, Isaac MT, Smith S, et al. Treatment of agitation caused by severe mental illness: data from the South London and Maudsley intensive care units trial evaluation (SLAMICUTE) study. European Neuropsychopharmacology 2006;16(Suppl 4):S420.
McEvoy 1994 {published data only}
-
- McEvoy JP. Efficacy of risperidone on positive features of schizophrenia. Journal of Clinical Psychiatry 1994;55(5 Suppl):18‐21. [MEDLINE: ] - PubMed
Mendelowitz 2004 {published data only}
-
- Mendelowitz AJ. The utility of intramuscular ziprasidone in the management of acute psychotic agitation. Annals of Clinical Psychiatry 2004;16(3):145‐54. [MEDLINE: ] - PubMed
NCT00189995 2005 {published data only}
-
- NCT00189995. Intramuscular clozapine in the management of aggression in schizophrenic patients. http://www.clinicaltrials.gov 2005.
NCT00631722 2008 {published data only}
-
- NCT00631722. Multicenter, open‐label, randomised, haloperidol‐controlled study to evaluate seroquel as mono‐therapy in the treatment of agitated symptoms in the patients with acute episode of schizophrenia. http://www.clinicaltrials.gov 2008.
NCT00797277 2008 {published data only}
-
- NCT00797277. IM olanzapine versus IM haloperidol plus lorazepam for acute agitation in schizophrenia. http://www.clinicaltrials.gov 2008.
Pan 2005 {published data only}
-
- Pan N, Yang X, Mei Q. Large dose of olanzapine in the ictus treatment of agitated symptoms of schizophrenia. Journal of Clinical Psychosomatic Diseases [临床心身疾病杂志] 2005;11(2):113‐5.
Pathiraja 1995 {published data only}
-
- Pathiraja AP, Diamond BI, Borison RL, Meibech RC, Anand R. Relationship between creatine phosphokinase, psychotic symptoms and novel antipsychotic drugs. Schizophrenia Research 1995;15(1‐2):160‐1.
Pedros 2004 {published data only}
-
- Pedros Rosello A, Tenias JM. Neuroleptics election and clinical outcomes in acute psychosis: A comparative study of risperidone versus other neuroleptics [Eleccion de neuroleptico y respuesta clinica en psicosis aguda: Un estudio comparativo de risperidona frente a otros neurolepticos]. Anales de Psiquiatria 2004;20(4):167‐71.
Pei 2009 {published data only}
-
- 裴树景, 杨红卫. Oral risperidone treatment of schizophrenia clinical observation of excited agitation [Google Translate] [利培酮口服液治疗精神分裂症兴奋激越临床观察]. Linchuang Jingshen Yixue Zazhi [临床精神医学杂志] 2009; Vol. 19, issue 6:405‐6.
Puech 1998 {published data only}
-
- Muller MJ, Wetzel H, Eich FX, Rein W, Puech A, Benkert O, Amisulpride Study Group. Dose‐related effects of amisulpride on five dimensions of psychopathology in patients with acute exacerbation of schizophrenia. Journal of Clinical Psychopharmacology 2002;22(6):554‐60. - PubMed
-
- Puech A, Fleurot O, Rein W, Amisulpride Study Group. Amisulpride, an atypical antipsychotic, in the treatment of acute episodes of schizophrenia: a dose‐ranging study vs. haloperidol. Acta Psychiatrica Scandinavica 1998;98(1):65‐72. [MEDLINE: ] - PubMed
Romeo 2009 {published data only}
-
- Romeo R, Knapp M, Tyrer P, Crawford M, Oliver‐Africano P. The treatment of challenging behaviour in intellectual disabilities: cost‐effectiveness analysis. Journal of Intellectual Disability Research 2009;53:633‐43. - PubMed
-
- Tyrer P. Neuroleptics in adults with aggressive challenging behaviour and intellectual disability. http://www.controlled‐trials.com 2003.
Simpson 2010 {published data only}
-
- Simpson G, Mohammad AR, Fauzia S, Campaneau M, Laska E, Ninah R, et al. A comparison of rapid titration quetiapine and haloperidol in agitated adults in an emergency setting. Proceedings of the 163rd Annual Meeting of the American Psychiatric Association; 2010 May 22‐26; New Orleans, LA 2010.
Singh 1981 {published data only}
-
- Singh MM, Kay SR, Pitman RK. Aggression control and structuring of social relations among recently admitted schizophrenics. Psychiatry Research 1981;5(2):157‐69. [MEDLINE: ] - PubMed
Smith 1974 {published data only}
-
- Smith GR, Taylor CW, Linkous P. Haloperidol versus thioridazine for the treatment of psychogeriatric patients: A double‐blind clinical trial. Psychosomatics 1974;15(3):134‐8.
Srinath 2010 {published data only}
-
- Srinath G, Shailaja B, Sai PG, Reddy KA. A comparative study of injection haloperidol and promethazine vs. injection lorazepam in controlling acute psychotic agitation. Indian Journal of Psychiatry 2010; Vol. 52:S21.
Teja 1975 {published data only}
-
- Teja JS, Grey WH, Clum JM, Warren C. Tranquilizers or anti‐depressants for chronic schizophrenics: A long term study. Australian and New Zealand Journal of Psychiatry 1975;9(4):241‐7. - PubMed
Thomas 1992 {published data only}
-
- Thomas HJ, Schwartz E, Petrilli R. Droperidol versus haloperidol for chemical restraint of agitated and combative patients. Annals of Emergency Medicine 1992;21(4):407‐13. [MEDLINE: ] - PubMed
TREC CG {published data only}
-
- Coutinho ES, Huf G, Allen MH, Adams CE. Physical restraints for agitated patients in psychiatric emergency hospitals in Rio de Janeiro, Brazil: a predictive model. Schizophrenia Bulletin 2005;31:220.
-
- Huf G. Rapid safe tranquillisation for acutely disturbed people attending public psychiatric emergency clinics in Rio de Janeiro. http://www.controlled‐trials.com 2002.
-
- Huf G, Coutinho ESF, Adams CE. TREC I. Background. Schizophrenia Research 2002;53(3 Suppl 1):187.
-
- Huf G, Coutinho ESF, Adams CE. TREC III. The protocol and progress of TREC. Schizophrenia Research 2002;53(3 Suppl 1):187.
Ungvari 1982 {published data only}
-
- Ungvari G, Petho B. High‐dose haloperidol therapy: Its effectiveness and a comparison with electroconvulsive treatment. Journal of Psychiatric Treatment and Evaluation 1982;4:279‐83.
Vaisanen 1981 {published data only}
-
- Vaisanen K, Viukari M, Rimon R, Raisanen P. Haloperidol, thioridazine and placebo in mentally subnormal patients‐serum levels and clinical effects. Acta Psychiatrica Scandinavica 1981;63(3):262‐71. [MEDLINE: ] - PubMed
Veser 2006 {published data only}
-
- Veser F, Zealburg J, Veser B, Zhu Y, Gharabawi G. Oral risperidone in the management of agitated behavior in emergency settings. European Neuropsychopharmacology 2002;12(Suppl 3):S313.
-
- Veser FH, Veser BD, McMullan JT, Zealberg J, Currier GW. Risperidone versus haloperidol, in combination with lorazepam, in the treatment of acute agitation and psychosis: a pilot, randomized, double‐blind, placebo‐controlled trial. Journal of Psychiatric Practice 2006;12(2):103‐8. [MEDLINE: ] - PubMed
Vives 2015 {published data only}
-
- Vives Vilagut R, Duno R, Planet N, Garcia G, Payes M, Marinosa M, et al. Testing the intranasal route for administration of haloperidol in emergency room: Generation of efficacy data to support clinical practice. Clinical Therapeutics 2015; Vol. 1:e152.
Wan 2005 {published data only}
-
- Wan Z, Zhong Z. Curative effect of risperidone with BDZs in the treatment of excitement and agitate with schizophrenia in acute phase. Heath Psychology Journal 2005;13(1):23‐4.
Wang 2004 {published data only}
-
- Wang G, Cai Z‐J, Wang L‐F. A multicenter study of risperidone treatment for acute agitation in patients with schizophrenia. Chinese Journal of Psychiatry 2004;37(2):88‐91.
Wang 2005 {published data only}
-
- Wang H, Guo X, Shen T. Safety and efficacy of quetiapine and haloperidol in the treatment of schizophrenia with excitement and agitation. Shanghai Archives of Psychiatry [上海精神医学] [上海精神醫學] 2005;17(5):271‐4.
Wyant 1990 {published data only}
-
- Wyant M, Diamond BI, O'Neal E, Sloan A, Borison RL. The use of midazolam in acutely agitated psychiatric patients. Psychopharmacology Bulletin 1990;26(1):126‐9. - PubMed
Zapletalek 1986 {published data only}
-
- Zapletalek M, Tuma I, Libiger J. Zetidoline compared with haloperidol in a double blind clinical study. Activitas Nervosa Superior 1986;28(1):31‐2.
References to studies awaiting assessment
Daniel 2006 {published data only}
-
- Daniel DG, Crandall D, Manos G, McQuade RD, Gutierrez‐Esteinou R, Pikalov AA, et al. Transitioning from intramuscular (IM) to oral aripiprazole in patients with schizophrenia. Proceedings of the 159th Annual Meeting of the American Psychiatric Association; 2006 May 20‐25; Toronto, Canada. 2006.
Davis 2008 {published data only}
-
- Davis JM, Wang B, He Y, Jin H, Hu Q, Zhang M. The emergency treatment of acutely agitated psychotic schizophrenia patient. International Journal of Neuropsychopharmacology 2008;11(Suppl 1):163.
Dubin 1985 {published data only}
-
- Dubin WR, Waxman HM, Weiss KJ, Ramchandani D, Tavani‐Petrone C. Rapid tranquilization: the efficacy of oral concentrate. Journal of Clinical Psychiatry 1985;46(11):475‐8. [MEDLINE: ] - PubMed
Esmailian 2015 {published data only}
Herrera 2005 {published data only}
-
- Herrera M. Double‐blind study with risperidone vs haloperidol in schizophrenic patients with agitation and/or aggression. Proceedings of the 8th World Congress of Psychiatry; 2005 Sep 10‐15; Cairo, Egypt. 2005.
Hsu 2010 {published data only}
-
- Hsu WY, Huang SS, Lee BS, Chiu NY. Comparison of intramuscular olanzapine, orally disintegrating olanzapine tablets, oral risperidone solution, and intramuscular haloperidol in the management of acute agitation in an acute care psychiatric ward in Taiwan. Journal of Clinical Psychopharmacology 2010;30(3):230‐4. - PubMed
Istikoglou 2010 {published data only}
-
- Istikoglou C, Vlavianou A, Foutsitzis D, Theodorakopoulos G, Polonifis C, Kanellos P, et al. Intramuscular aripiprazole versus injectable haloperidol in treatment of acute psychotic agitation. European Neuropsychopharmacology 2010;20(3):S469‐70.
Kong 2009 {published data only}
-
- Kong BG, Shim JC, Lee SK. Comparison of the effects of intramuscular antipsychotics injection in the treatment of acute agitation in psychosis. European Neuropsychopharmacology 2009; Vol. 19:S525‐S6.
Lasic 2006 {published data only}
-
- Lasic D, Rubesa G, Dodig G, Katavic Z, Glavina T, Zuljan‐Cvitanovic M. Risperidone oral solution, therapeutic possibility in treatment of acute and agitated psychotic patients. European Neuropsychopharmacology 2006;16(Suppl 4):S445.
Li 2007a {published data only}
-
- Li C, Zhu S, Wang H, Chen H, Yu Y, Liu D, et al. Safety and efficacy of clonazepam, haloperidol and haloperidol combined with clonazepam in the [氯硝西泮、氟哌啶醇及其联合治疗对精神分裂症激越症状疗效的比较]. Shanghai Archives of Psychiatry [上海精神医学] [上海精神醫學] 2007; Vol. 19, issue 3:150‐2.
Lu 2006 {published data only}
-
- Lu D, Ning J, Zhong X. Clinical evaluation on treatment of acute‐agitation in schizophrenia with zipraside injection [甲磺酸齐拉西酮注射液治疗精神分裂症急性激越症状的临床评价]. China Pharmaceuticals 2006;15(4):48‐9.
NCT00859872 2009 {published data only}
-
- NCT00859872. Efficacy and safety of risperidone oral solution combination clonazepam versus haloperidol intramuscular (IM) injection for treatment of acute psychotic agitation in schizophrenia. http://www.clinicaltrials.gov 2009.
NCT00866645 2009 {published data only}
-
- NCT00866645. A study to evaluate the efficacy and safety of intramuscular levosulpiride in patients with agitation of schizophrenia. http://www.clinicaltrials.gov 2009.
Shim 2009 {published data only}
-
- Shim JC, Kong BG, Lee SG, Kim YH. The effect of intramuscular olanzapine in the treatment of acute agitation in patient with psychotic symptoms. European Neuropsychopharmacology 2009; Vol. 19:S512‐S3.
Si 2009 {published data only}
-
- Si T, Zhong HJ, Yun'Ai S, Qin TM, Qing LK, De YP, et al. A multi‐site,open‐label, randomised, haloperidol‐controlled study to evaluate quetiapine as monotherapy in the treatment of agitated acute schizophrenia. Proceedings of the 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16‐21; San Francisco, CA 2009.
Slotnick 1971 {published data only}
-
- Slotnick VB. Management of the acutely agitated psychiatric patient with parental neuroleptics: The comparitive symptom effectiveness profiles of haloperidol and chlorpromazine. Proceedings of the 5th World Congress of Psychiatry; 1971 Nov 28 ‐ Dec 4; Mexico City, Mexico. 1971.
Smythies 1982 {published data only}
-
- Smythies JR, Hain J, Crews E, Grayson G. A clinical trial of injectable molindone (Moban(R)). Current Therapeutic Research, Clinical and Experimental 1982;32(5):752‐6.
Vasquez‐Gomez 2001 {published data only}
-
- Vasquez‐Gomez F. The use of midazolam for control of acutely agitated outpatients in psychiatry emergency. Proceedings of the 7th World Congress of Biological Psychiatry; 2001 Jul 1‐6; Berlin, Germany. 2001.
Wang 2005a {published data only}
-
- Wang R‐C, Li L‐H, Zhao Y‐C. Ziprasidone made in China and haloperidol in treating acute onset of schizophrenia: a randomized, single blinded, double modelling, parallel control study. Chinese Journal of Clinical Rehabilitation 2005; Vol. 9, issue 44:83‐5.
Wang 2007 {published data only}
-
- Wang J‐C, Xu X‐F, Ouyang H, Li W‐Y. The second phase clinical trials of intramuscular ziprasidone for the treatment of acute psychotic agitation of the schizophrenia [注射用甲磺酸齐拉西酮治疗精神分裂症兴奋躁动状态的Ⅱ期临床试验]. Nervous Diseases and Mental Health [神经疾病与精神卫生] 2007;7(5):364‐70.
Yildiz 2003 {published data only}
-
- Yildiz A, Turgay A, Alpay M, Sachs GS. Risperidone versus haloperidol in adult psychiatric emergencies. Proceedings of the 156th Annual Meeting of the American Psychiatric Association; 2003 May 17‐22; San Francisco, California, USA 2003:Nr259.
Zhang 2012 {published data only}
-
- 张庆娥, 王刚, 张玲, 王雪, 罗炯, 路亚洲, et al. Control study of acute agitation in schizophrenia risperidone oral solution merger clonazepam tablets treatment [Google Translate] [利培酮口服液合并氯硝西泮片治疗精神分裂症急性激越的对照研究]. 中华医学会第十次全国精神医学学术会议 2012; Vol. 22, issue 02:89‐91.
叶萌, 2009 {published data only}
-
- 叶萌, 房茂胜, 李乐华, 赵靖平. Domestic ziprasidone mesylate injection in the treatment of acute agitation of schizophrenia [国产甲磺酸齐拉西酮注射液治疗精神分裂症急性激越症状疗效观察]. Journal of the Xinxiang Medical College [新乡医学院学报] 2009; Vol. 26, issue 1:63‐5.
周德祥, 2012 {published data only}
-
- 周德祥, 蒋幸衍, 徐清, 方馨怡, 夏鸣华, 陆雅娜. 利培酮口服液合并氯硝西泮治疗精神分裂症兴奋激越症状的随机对照研究. 中国健康心理学杂志 2012; Vol. 8:1123‐4.
References to ongoing studies
NCT00838032 2009 {published data only}
-
- NCT00838032. Pilot study to evaluate the efficacy and safety of quetiapine fumarate instant‐release (seroquel ir) in controlling agitation and aggressive symptoms in the acute treatment of patients with schizophrenia. http://www.clinicaltrials.gov 2009.
Additional references
Adams 2013
Ahmed 2010
Ahmed 2011
Altman 1996
APA 2006
-
- American Psychiatric Association. Practice Guideline for the Treatment of Patients With Schizophrenia. 2nd Edition. Washington DC: American Psychiatric Association, 2006. [DOI: 10.1176/appi.books.9780890423363.45859] - DOI
Barnes 1989
-
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. - PubMed
Belgamwar 2005
Berk 2004
Bland 1997
BNF 2011
-
- British National Formulary. British National Formulary. 61. NHS, London: Pharmaceutical Press, 2011.
Boissel 1999
-
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Apercu sur la problematique des indices d'efficacite therapeutique, 3: comparaison des indices et utilisation. Groupe d'etude des indices d'efficacite]. Therapie 1999;54(4):405‐11. [PUBMED: 10667106] - PubMed
Choudhury 2011
-
- Choudhury R, Dewsbery M, Williams K, Hovey N. Audit of the rapid tranquilization policy in the 2gether NHS foundation trust. Journal of Psychiatric Intensive Care 2011;7(1):55‐61.
Corrigan 1989
-
- Corrigan JD. Development of a scale for assessment of agitation following traumatic brain injury. Journal of Clinical and Experimental Neuropsychology 1989;11(2):261‐77. - PubMed
Deeks 2000
-
- Deeks J. Issues in the selection for meta‐analyses of binary data. Proceedings of the 8th International Cochrane Colloquium; 2000 Oct 25‐28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Divine 1992
-
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. - PubMed
Donner 2002
-
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. - PubMed
Douglas‐Hall 2015
Du 2017
Easterbrook 1991
-
- Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet 1991;337(8746):867‐72. [PUBMED: 1672966] - PubMed
Egger 1997
Elbourne 2002
-
- Elbourne D, Altman DG, Higgins JPT, Curtina F, Worthingtond HV, Vaile A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(7):7‐10. - PubMed
Gillies 2013
Glazer 1999
-
- Glazer WM. Does loxapine have "atypical" properties? Clinical evidence. Journal of Clinical Psychiatry 1999;60(Suppl 10):42‐6. [PUBMED: 10340686] - PubMed
Gulliford 1999
-
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. - PubMed
Guy 1976
-
- Guy W. Clinical Global Impression. ECDEU Assessment Manual for Psychopharmacology. revised. National Institute of Mental Health, 1976:217‐21.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Huf 2016
Isbister 2010
-
- Isbister GK, Calver LA, Page CB, Stokes B, Bryant JL, Downes MA. Randomized controlled trial of intramuscular droperidol versus midazolam for violence and acute behavioral disturbance: the DORM study. Annals of Emergency Medicine 2010;56(4):392‐401. [PUBMED: 20868907] - PubMed
Janssen‐Cilag Ltd 2010
-
- Janssen‐Cilag Ltd. Haldol (haloperidol) 5mg/ml Injection. Summary of product characteristics. http://www.medicines.org.uk/emc/medicine/7267/spc/haldol+injection/ (accessed 3 June 2011).
Jayakody 2012
Kay 1986
-
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi‐Health Systems, 1986.
Kay 1987
-
- Kay SR, Flszbein A, Opfer LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Psychological Reports 1962;10:799‐812. - PubMed
Khokhar 2016
Khushu 2016
Leucht 2005
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366‐71. [PUBMED: 16199797] - PubMed
Leucht 2005a
-
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean?. Schizophrenia Research 2005;79(2‐3):231‐8. [PUBMED: 15982856] - PubMed
Leucht 2007
Lexchin 2003
Lieberman 2005
-
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine 2005;353(12):1209‐23. [PUBMED: 16172203] - PubMed
López‐Munoz 2009
-
- López‐Munoz F, Alamo C. The consolidation of neuroleptic therapy: Janssen, the discovery of haloperidol and its introduction into clinical practice. Brain Research Bulletin 2009;79:130–41. - PubMed
Marshall 2000
-
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. - PubMed
Moher 2001
-
- Moher D, Schulz KF, Altman DG. The CONSORT statement:revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. - PubMed
Mohr 2005
-
- Mohr P, Pecenak J, Svestka J, Swingler D, Treuer T. Treatment of acute agitation in psychotic disorders. Neuro Endocrinology Letters 2005;26(4):327‐35. [PUBMED: 16136016] - PubMed
Montoya 2011
-
- Montoya A, Valladares A, Lizán L, San L, Escobar R, Paz S. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS‐EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Quality Life Outcomes 2011;9:18. - PMC - PubMed
Muralidharan 2006
NHS 2009
-
- National Health Service. The prescribing preview report on antipsychotic drugs, available to general practitioners in August 2009, is reproduced here for readers with an interest in patterns and trends of prescribing. Impact: Prescribing and Dispensing Newsletter. http://www.nhsbsa.nhs.uk/PrescriptionServices/Documents/PrescriptionServ... (accessed 2nd June 2011):4‐6.
NICE 2005
-
- National Institute for Clinical Excellence. The short term‐management of disturbed/violent behaviour in inpatient psychiatric settings and emergency departments. Vol. 25, London: NICE, 2005.
NICE 2015
-
- National Institute for Clinical Excellence. Violence and aggression: short‐term management in mental health, health and community settings. nice.org.uk/guidance/ng10 2015.
Olkkola 2008
-
- Olkkola KT, Ahonen J. Midazolam and other benzodiazepines. Handbook of Experimental Pharmacology 2008;182:335‐60. [PUBMED: 18175099] - PubMed
Overall 1962
-
- Overall JE, Gorham DR. The brief psychiatric rating scale. Psychological Reports 1962;10:799‐812.
Parker 2010
-
- Parker C, Khwaja MG. What is new in rapid tranquillisation?. Journal of Psychiatric Intensive Care 2010;7(2):1‐11.
Pereira 2007
-
- Pereira S, Fleischhacker W, Allen M. Management of behavioural emergencies. Journal of Psychiatric Intensive Care 2007;2(2):71–83.
Pratt 2008
-
- Pratt P, Chandler‐Oatts J, Nelstrop L, Branford D, Periera S, Johnston S. Establishing gold standard approaches to rapid tranquillisation: A review and discussion of the evidence of the safety and efficacy of medications used. Journal of Psychiatric Intensive Care 2008;4(1‐2):43‐57.
Reid 2003
-
- Reid G, Hughson M. Droperidol dropped; consultants not consulted: A survey of the practice of rapid tranquillisation by consultant psychiatrists in the west of Scotland. Psychiatric Bulletin 2003;27:301‐4.
Sailas 2000
Schleifer 2011
-
- Schleifer JJ. Management of acute agitation in psychosis: an evidence‐based approach in the USA. Advances in Psychiatric Treatment 2011;17:91‐100.
Schünemann 2008
-
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration, 2008:359‐83.
Simpson 1970
-
- Simpson GM, Angus JSW. A rating scale for extrapyramidal side effects. Acta Psychiatri Scandinavica. Supplementum 1970;212:11‐9. - PubMed
Thorpe 2009
-
- Thorpe KE, Zwarenstein M, Oxman AD, Treweek S, Furberg CD, Altman DG, et al. A pragmatic‐explanatory continuum indicator summary (PRECIS): a tool to help trial designers. Journal of Clinical Epidemiology 2009;62(5):464‐75. [PUBMED: 19348971] - PubMed
TREC 2003
Ukoumunne 1999
-
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: A systematic review. Health Technology Assessment 1999;3(5):1‐75. - PubMed
Vangala 2012
-
- Vangala R, Ahmed U, Ahmed R. Loxapine inhaler for psychosis‐induced aggression. Cochrane Database of Systematic Reviews 2012.
WHO 2011
-
- World Health Organization. WHO Essential Medicines List. http://whqlibdoc.who.int/hq/2011/a95053_eng.pdf (accessed 2nd June 2011).
WHO 2015
-
- World Health Organization. WHO Model List of Essential Medicines. http://www.who.int/medicines/publications/essentialmedicines/EML_2015_FI... 2015.
Wilkie 2012
-
- Wilkie F, Fenton M. Quetiapine for psychosis‐induced aggression or agitation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009801] - DOI
Xia 2009
-
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254‐7.
Xiberas 2001
-
- Xiberas X, Martinot JL, Mallet L, Artiges E, Loc'h C, Maziere B, et al. Extrastriatal and striatal D2 dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. British Journal of Psychiatry 2001;179:503‐8. - PubMed
Yudofsky 1986
-
- Yudofsky SC, Silver JM, Jackson W, Endicott J, Williams D. The Overt Aggression Scale for the objective rating of verbal and physical aggression. American Journal of Psychiatry 1986;143:35‐9. - PubMed
Yudofsky 1997
-
- Yudofsky SC, Kopecky HJ, Kunik M, Silver JM, Endicott J. The Overt Agitation Severity Scale for the objective rating of agitation. Journal of Neuropsychiatry 1997;9(4):541‐8. - PubMed
References to other published versions of this review
Powney 2011
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

