The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore
- PMID: 28758328
- PMCID: PMC5595682
- DOI: 10.1111/acel.12650
The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore
Abstract
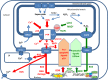
Excessive production of mitochondrial reactive oxygen species (mROS) is strongly associated with mitochondrial and cellular oxidative damage, aging, and degenerative diseases. However, mROS also induces pathways of protection of mitochondria that slow aging, inhibit cell death, and increase lifespan. Recent studies show that the activation of the mitochondrial permeability transition pore (mPTP), which is triggered by mROS and mitochondrial calcium overloading, is enhanced in aged animals and humans and in aging-related degenerative diseases. mPTP opening initiates further production and release of mROS that damage both mitochondrial and nuclear DNA, proteins, and phospholipids, and also releases matrix NAD that is hydrolyzed in the intermembrane space, thus contributing to the depletion of cellular NAD that accelerates aging. Oxidative damage to calcium transporters leads to calcium overload and more frequent opening of mPTP. Because aging enhances the opening of the mPTP and mPTP opening accelerates aging, we suggest that mPTP opening drives the progression of aging. Activation of the mPTP is regulated, directly and indirectly, not only by the mitochondrial protection pathways that are induced by mROS, but also by pro-apoptotic signals that are induced by DNA damage. We suggest that the integration of these contrasting signals by the mPTP largely determines the rate of cell aging and the initiation of cell death, and thus animal lifespan. The suggestion that the control of mPTP activation is critical for the progression of aging can explain the conflicting and confusing evidence regarding the beneficial and deleterious effects of mROS on health and lifespan.
Keywords: NAD; aging; calcium; mitochondria; permeability transition; reactive oxygen species.
© 2017 The Authors. Aging Cell published by the Anatomical Society and John Wiley & Sons Ltd.
Figures
Similar articles
-
The effect of permeability transition pore opening on reactive oxygen species production in rat brain mitochondria.Ukr Biokhim Zh (1999). 2011 Nov-Dec;83(6):46-55. Ukr Biokhim Zh (1999). 2011. PMID: 22364018
-
The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury.J Mol Cell Cardiol. 2015 Jan;78:129-41. doi: 10.1016/j.yjmcc.2014.08.018. Epub 2014 Aug 30. J Mol Cell Cardiol. 2015. PMID: 25179911 Review.
-
Review: Chemical Pathology of Homocysteine VI. Aging, Cellular Senescence, and Mitochondrial Dysfunction.Ann Clin Lab Sci. 2018 Sep;48(5):677-687. Ann Clin Lab Sci. 2018. PMID: 30373877 Review.
-
Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability.Sci Rep. 2017 Sep 5;7(1):10492. doi: 10.1038/s41598-017-10673-8. Sci Rep. 2017. PMID: 28874733 Free PMC article.
-
Cyclosporine A normalizes mitochondrial coupling, reactive oxygen species production, and inflammation and partially restores skeletal muscle maximal oxidative capacity in experimental aortic cross-clamping.J Vasc Surg. 2013 Apr;57(4):1100-1108.e2. doi: 10.1016/j.jvs.2012.09.020. Epub 2013 Jan 18. J Vasc Surg. 2013. PMID: 23332985
Cited by
-
Dexmedetomidine ameliorates acute kidney injury by regulating mitochondrial dynamics via the α2-AR/SIRT1/PGC-1α pathway activation in rats.Mol Med. 2024 Oct 25;30(1):184. doi: 10.1186/s10020-024-00964-y. Mol Med. 2024. PMID: 39455916 Free PMC article.
-
Mitochondrial Permeability Uncouples Elevated Autophagy and Lifespan Extension.Cell. 2019 Apr 4;177(2):299-314.e16. doi: 10.1016/j.cell.2019.02.013. Epub 2019 Mar 28. Cell. 2019. PMID: 30929899 Free PMC article.
-
Cyclophilin D, regulator of the mitochondrial permeability transition, impacts bone development and fracture repair.Bone. 2024 Dec;189:117258. doi: 10.1016/j.bone.2024.117258. Epub 2024 Sep 18. Bone. 2024. PMID: 39299628
-
Untargeted Metabolomics Reveals Acylcarnitines as Major Metabolic Targets of Resveratrol in Breast Cancer Cells.Metabolites. 2025 Apr 5;15(4):250. doi: 10.3390/metabo15040250. Metabolites. 2025. PMID: 40278380 Free PMC article.
-
Age-Related Changes in Bone-Marrow Mesenchymal Stem Cells.Cells. 2021 May 21;10(6):1273. doi: 10.3390/cells10061273. Cells. 2021. PMID: 34063923 Free PMC article.
References
-
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA Jr, Jonas EA (2014) An uncoupling channel within the c‐subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc. Natl Acad. Sci. USA 111, 10580–10585. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

