Tree shrew, a potential animal model for hepatitis C, supports the infection and replication of HCV in vitro and in vivo
- PMID: 28758632
- PMCID: PMC5656785
- DOI: 10.1099/jgv.0.000869
Tree shrew, a potential animal model for hepatitis C, supports the infection and replication of HCV in vitro and in vivo
Abstract
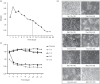
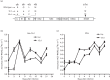
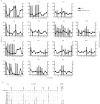
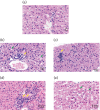
The tree shrew (Tupaia belangeri chinensis), a small animal widely distributed in Southeast Asia and southwest China, has the potential to be developed as an animal model for hepatitis C. To determine the susceptibility of the tree shrew to hepatitis C virus (HCV) infection in vitro and in vivo, a well-established HCV, produced from the J6/JFH1-Huh7.5.1 culture system, was used to infect cultured primary tupaia hepatocytes (PTHs) and tree shrews. The in vitro results showed that HCV genomic RNA and HCV-specific nonstructural protein 5A (NS5A) could be detected in the PTH cell culture from days 3-15 post-infection, although the viral load was lower than that observed in Huh7.5.1 cell culture. The occurrence of five sense mutations [S391A, G397A, L402F and M405T in the hypervariable region 1 (HVR1) of envelope glycoprotein 2 and I2750M in NS5B] suggested that HCV undergoes genetic evolution during culture. Fourteen of the 30 experimental tree shrews (46.7 %) were found to be infected, although the HCV viremia was intermittent in vivo. A positive test for HCV RNA in liver tissue provided stronger evidence for HCV infection and replication in tree shrews. The results of an immunohistochemistry assay also demonstrated the presence of four HCV-specific proteins (Core, E2, NS3/4 and NS5A) in the hepatocytes of infected tree shrews. The pathological changes observed in the liver tissue of infected tree shrews could be considered to be representative symptoms of mild hepatitis. These results revealed that the tree shrew can be used as an animal model supporting the infection and replication of HCV in vitro and in vivo.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

