Parathyroid hormone regulates fates of murine osteoblast precursors in vivo
- PMID: 28758904
- PMCID: PMC5669555
- DOI: 10.1172/JCI91699
Parathyroid hormone regulates fates of murine osteoblast precursors in vivo
Abstract
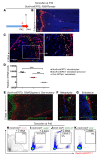
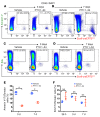
Teriparatide, a recombinant form of parathyroid hormone (PTH), is the only approved treatment for osteoporosis that increases the rate of bone formation. Teriparatide increases osteoblast numbers by suppressing osteoblast apoptosis and activating bone-lining cells. No direct evidence for teriparatide's actions on early cells of the osteoblast lineage has been demonstrated. Here, we have employed a lineage-tracing strategy that uses a tamoxifen-dependent, promoter-driven cre to mark early cells of the osteoblast lineage in adult mice. We show that teriparatide increases the numbers of osteoblast precursors and drives their differentiation into mature osteoblasts. Unexpectedly, following withdrawal of teriparatide therapy, bone marrow adipocytes increased dramatically in number. Some of these adipocytes derived from cells marked by Sox9-cre expression weeks earlier. Continued therapy with teriparatide prevented the appearance of adipocytes. Selective, inducible deletion of the PTH receptor in Sox9-cre cells demonstrated that PTH receptor expression is required for teriparatide-mediated increases in early osteoblast precursors. The increase in early precursors after teriparatide administration was associated with robust suppression of precursor apoptosis without affecting their rate of proliferation. Thus, teriparatide increases the numbers of early cells of the osteoblast lineage, hastens their differentiation into osteoblasts, and suppresses their differentiation into adipocytes in vivo.
Conflict of interest statement
Figures




References
-
- Heaney RP. Vitamin D and parathyroid hormone. In: Bilezikian JP, Marcus R, Levine MA, Marcocci C, Silverberg SJ, Potts JT, eds. The Parathyroids:Basic and Clinical Concepts. Cambridge, MA: Academic Press; 2015:633–640.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

