Effect of a Particulate and a Putty-Like Tricalcium Phosphate-Based Bone-grafting Material on Bone Formation, Volume Stability and Osteogenic Marker Expression after Bilateral Sinus Floor Augmentation in Humans
- PMID: 28758916
- PMCID: PMC5618282
- DOI: 10.3390/jfb8030031
Effect of a Particulate and a Putty-Like Tricalcium Phosphate-Based Bone-grafting Material on Bone Formation, Volume Stability and Osteogenic Marker Expression after Bilateral Sinus Floor Augmentation in Humans
Abstract
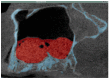
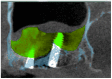
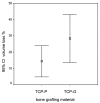
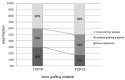
This study examines the effect of a hyaluronic acid (HyAc) containing tricalcium phosphate putty scaffold material (TCP-P) and of a particulate tricalcium phosphate (TCP-G) graft on bone formation, volume stability and osteogenic marker expression in biopsies sampled 6 months after bilateral sinus floor augmentation (SFA) in 7 patients applying a split-mouth design. 10% autogenous bone chips were added to the grafting material during surgery. The grain size of the TCP granules was 700 to 1400 µm for TCP-G and 125 to 250 µm and 500 to 700 µm (ratio 1:1) for TCP-P. Biopsies were processed for immunohistochemical analysis of resin-embedded sections. Sections were stained for collagen type I (Col I), alkaline phosphatase (ALP), osteocalcin (OC) and bone sialoprotein (BSP). Furthermore, the bone area and biomaterial area fraction were determined histomorphometrically. Cone-beam CT data recorded after SFA and 6 months later were used for calculating the graft volume at these two time points. TCP-P displayed more advantageous surgical handling properties and a significantly greater bone area fraction and smaller biomaterial area fraction. This was accompanied by significantly greater expression of Col I and BSP and in osteoblasts and osteoid and a less pronounced reduction in grafting volume with TCP-P. SFA using both types of materials resulted in formation of sufficient bone volume for facilitating stable dental implant placement with all dental implants having been in function without any complications for 6 years. Since TCP-P displayed superior surgical handling properties and greater bone formation than TCP-G, without the HyAc hydrogel matrix having any adverse effect on bone formation or graft volume stability, TCP-P can be regarded as excellent grafting material for SFA in a clinical setting. The greater bone formation observed with TCP-P may be related to the difference in grain size of the TCP granules and/or the addition of the HyAc.
Keywords: bioactive ceramics; bone formation; bone-grafting materials; hard tissue histology; immunohistochemical analysis; osteogenesis; osteogenic markers; sinus floor augmentation; split-mouth design; tricalcium phosphate putty scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Performance of β-tricalcium phosphate granules and putty, bone grafting materials after bilateral sinus floor augmentation in humans.Biomaterials. 2014 Mar;35(10):3154-63. doi: 10.1016/j.biomaterials.2013.12.068. Epub 2014 Jan 16. Biomaterials. 2014. PMID: 24439419
-
Effect of beta-tricalcium phosphate particles with varying porosity on osteogenesis after sinus floor augmentation in humans.Biomaterials. 2008 May;29(14):2249-58. doi: 10.1016/j.biomaterials.2008.01.026. Epub 2008 Mar 4. Biomaterials. 2008. PMID: 18289665
-
Effect of Tricalcium Phosphate Foam and Paste Bone Grafting Materials Designed for Improved Surgical Handling on Osteogenesis in a Sheep Scapula Model.J Biomed Mater Res B Appl Biomater. 2025 Apr;113(4):e35561. doi: 10.1002/jbm.b.35561. J Biomed Mater Res B Appl Biomater. 2025. PMID: 40095749
-
Insights into Sinus-Lift Bone Grafting Materials: What's Changed?J Funct Biomater. 2025 Apr 7;16(4):133. doi: 10.3390/jfb16040133. J Funct Biomater. 2025. PMID: 40278241 Free PMC article. Review.
-
Bicalcium Phosphate as an Asset in Regenerative Therapy.Cureus. 2023 Aug 24;15(8):e44079. doi: 10.7759/cureus.44079. eCollection 2023 Aug. Cureus. 2023. PMID: 37750142 Free PMC article. Review.
Cited by
-
Advancements in Hyaluronic Acid Effect in Alveolar Ridge Preservation: A Narrative Review.Diagnostics (Basel). 2025 Jan 8;15(2):137. doi: 10.3390/diagnostics15020137. Diagnostics (Basel). 2025. PMID: 39857021 Free PMC article. Review.
-
Osteogenic Effect of a Bioactive Calcium Alkali Phosphate Bone Substitute in Humans.Bioengineering (Basel). 2023 Dec 11;10(12):1408. doi: 10.3390/bioengineering10121408. Bioengineering (Basel). 2023. PMID: 38135999 Free PMC article.
-
A tissue engineered 3D printed calcium alkali phosphate bioceramic bone graft enables vascularization and regeneration of critical-size discontinuity bony defects in vivo.Front Bioeng Biotechnol. 2023 Jun 15;11:1221314. doi: 10.3389/fbioe.2023.1221314. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37397960 Free PMC article.
References
-
- Aghaloo T.L., Moy P.K. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int. J. Oral Maxillofac. Implant. 2007;22:49–70. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

