Research Progress in the Modification of Quercetin Leading to Anticancer Agents
- PMID: 28758919
- PMCID: PMC6152094
- DOI: 10.3390/molecules22081270
Research Progress in the Modification of Quercetin Leading to Anticancer Agents
Abstract
The flavonoid quercetin (3,3',4',5,7-pentahydroxyflavone) is widely distributed in plants, foods, and beverages. This polyphenol compound exhibits varied biological actions such as antioxidant, radical-scavenging, anti-inflammatory, antibacterial, antiviral, gastroprotective, immune-modulator, and finds also application in the treatment of obesity, cardiovascular diseases and diabetes. Besides, quercetin can prevent neurological disorders and exerts protection against mitochondrial damages. Various in vitro studies have assessed the anticancer effects of quercetin, although there are no conclusive data regarding its mode of action. However, low bioavailability, poor aqueous solubility as well as rapid body clearance, fast metabolism and enzymatic degradation hamper the use of quercetin as therapeutic agent, so intense research efforts have been focused on the modification of the quercetin scaffold to obtain analogs with potentially improved properties for clinical applications. This review gives an overview of the developments in the synthesis and anticancer-related activities of quercetin derivatives reported from 2012 to 2016.
Keywords: anticancer; cell proliferation; cytotoxicity; methoxyflavones; multi-drug resistance (MDR); quercetin; quercetin derivatives.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
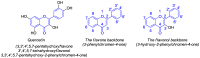
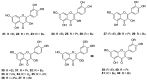

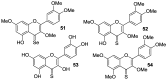
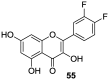
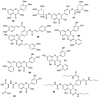
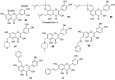
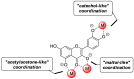
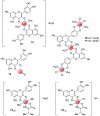
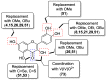
Similar articles
-
Structural Design, Synthesis and Antioxidant, Antileishmania, Anti-Inflammatory and Anticancer Activities of a Novel Quercetin Acetylated Derivative.Molecules. 2021 Nov 17;26(22):6923. doi: 10.3390/molecules26226923. Molecules. 2021. PMID: 34834016 Free PMC article.
-
Synthesis and biological evaluation of quercetin and resveratrol peptidyl derivatives as potential anticancer and antioxidant agents.Amino Acids. 2019 Feb;51(2):319-329. doi: 10.1007/s00726-018-2668-6. Epub 2018 Nov 3. Amino Acids. 2019. PMID: 30392096
-
Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes.Artif Cells Nanomed Biotechnol. 2016;44(1):128-34. doi: 10.3109/21691401.2014.926456. Epub 2014 Jun 24. Artif Cells Nanomed Biotechnol. 2016. PMID: 24959911
-
Quercetin and its derivatives: synthesis, pharmacological uses with special emphasis on anti-tumor properties and prodrug with enhanced bio-availability.Anticancer Agents Med Chem. 2009 Feb;9(2):138-61. doi: 10.2174/187152009787313855. Anticancer Agents Med Chem. 2009. PMID: 19199862 Review.
-
Quercetin-Amino Acid Conjugates are Promising Anti-Cancer Agents in Drug Discovery Projects.Mini Rev Med Chem. 2020;20(2):107-122. doi: 10.2174/1389557519666191009152007. Mini Rev Med Chem. 2020. PMID: 31595850 Review.
Cited by
-
Structural Design, Synthesis and Antioxidant, Antileishmania, Anti-Inflammatory and Anticancer Activities of a Novel Quercetin Acetylated Derivative.Molecules. 2021 Nov 17;26(22):6923. doi: 10.3390/molecules26226923. Molecules. 2021. PMID: 34834016 Free PMC article.
-
Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications-A Review.Pharmaceuticals (Basel). 2023 Nov 20;16(11):1631. doi: 10.3390/ph16111631. Pharmaceuticals (Basel). 2023. PMID: 38004496 Free PMC article. Review.
-
Functions of polyphenols and its anticancer properties in biomedical research: a narrative review.Transl Cancer Res. 2020 Dec;9(12):7619-7631. doi: 10.21037/tcr-20-2359. Transl Cancer Res. 2020. PMID: 35117361 Free PMC article. Review.
-
Quercetin as a Promising Antiprotozoan Phytochemical: Current Knowledge and Future Research Avenues.Biomed Res Int. 2024 Feb 29;2024:7632408. doi: 10.1155/2024/7632408. eCollection 2024. Biomed Res Int. 2024. PMID: 38456097 Free PMC article. Review.
-
Biochemical characterization and inhibition of thermolabile hemolysin from Vibrio parahaemolyticus by phenolic compounds.PeerJ. 2021 Jan 6;9:e10506. doi: 10.7717/peerj.10506. eCollection 2021. PeerJ. 2021. PMID: 33505784 Free PMC article.
References
-
- Scalbert A., Williamson G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000;130:2073S–2085S. - PubMed
-
- Materska M. Quercetin and its Derivatives: Chemical Structure and Bioactivity—A Review. Pol. J. Food Nutr. Sci. 2008;58:407–413.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

