Nitrogenous Derivatives of Phosphorus and the Origins of Life: Plausible Prebiotic Phosphorylating Agents in Water
- PMID: 28758921
- PMCID: PMC5617957
- DOI: 10.3390/life7030032
Nitrogenous Derivatives of Phosphorus and the Origins of Life: Plausible Prebiotic Phosphorylating Agents in Water
Abstract
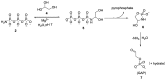
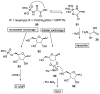
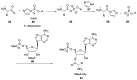
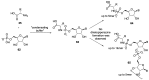
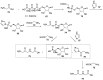
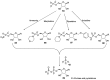
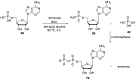
Phosphorylation under plausible prebiotic conditions continues to be one of the defining issues for the role of phosphorus in the origins of life processes. In this review, we cover the reactions of alternative forms of phosphate, specifically the nitrogenous versions of phosphate (and other forms of reduced phosphorus species) from a prebiotic, synthetic organic and biochemistry perspective. The ease with which such amidophosphates or phosphoramidate derivatives phosphorylate a wide variety of substrates suggests that alternative forms of phosphate could have played a role in overcoming the "phosphorylation in water problem". We submit that serious consideration should be given to the search for primordial sources of nitrogenous versions of phosphate and other versions of phosphorus.
Keywords: (di)amidophosphate; nitrogen-phosphorus derivatives; origins of life; phosphoramidates; prebiotic phosphorylation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
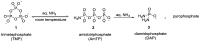

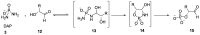
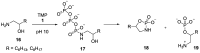

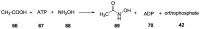

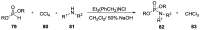




Similar articles
-
Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions.Nat Chem. 2018 Feb;10(2):212-217. doi: 10.1038/nchem.2878. Epub 2017 Nov 6. Nat Chem. 2018. PMID: 29359747 Free PMC article.
-
A Chemist's Perspective on the Role of Phosphorus at the Origins of Life.Life (Basel). 2017 Jul 13;7(3):31. doi: 10.3390/life7030031. Life (Basel). 2017. PMID: 28703763 Free PMC article. Review.
-
Geochemical Sources and Availability of Amidophosphates on the Early Earth.Angew Chem Int Ed Engl. 2019 Jun 11;58(24):8151-8155. doi: 10.1002/anie.201903808. Epub 2019 May 8. Angew Chem Int Ed Engl. 2019. PMID: 30989779
-
Sources of Nitrogen-, Sulfur-, and Phosphorus-Containing Feedstocks for Prebiotic Chemistry in the Planetary Environment.Life (Basel). 2022 Aug 19;12(8):1268. doi: 10.3390/life12081268. Life (Basel). 2022. PMID: 36013447 Free PMC article. Review.
-
A Stark Contrast to Modern Earth: Phosphate Mineral Transformation and Nucleoside Phosphorylation in an Iron- and Cyanide-Rich Early Earth Scenario.Angew Chem Int Ed Engl. 2019 Nov 18;58(47):16981-16987. doi: 10.1002/anie.201908272. Epub 2019 Oct 4. Angew Chem Int Ed Engl. 2019. PMID: 31460687
Cited by
-
Cyclotriphosphate: A Brief History, Recent Developments, and Perspectives in Synthesis.Chemistry. 2020 Feb 21;26(11):2298-2308. doi: 10.1002/chem.201904433. Epub 2019 Dec 11. Chemistry. 2020. PMID: 31637774 Free PMC article.
-
Phosphorylation of nucleosides by P-N bond species generated from prebiotic reduced phosphorus sources.Commun Chem. 2025 Jun 17;8(1):187. doi: 10.1038/s42004-025-01577-0. Commun Chem. 2025. PMID: 40523969 Free PMC article.
-
Experimentally investigating the origin of DNA/RNA on early Earth.Nat Commun. 2018 Dec 12;9(1):5175. doi: 10.1038/s41467-018-07212-y. Nat Commun. 2018. PMID: 30538231 Free PMC article.
-
Phosphorylation, oligomerization and self-assembly in water under potential prebiotic conditions.Nat Chem. 2018 Feb;10(2):212-217. doi: 10.1038/nchem.2878. Epub 2017 Nov 6. Nat Chem. 2018. PMID: 29359747 Free PMC article.
-
Carbamoyl phosphate and its substitutes for the uracil synthesis in origins of life scenarios.Sci Rep. 2021 Sep 29;11(1):19356. doi: 10.1038/s41598-021-98747-6. Sci Rep. 2021. PMID: 34588537 Free PMC article.
References
-
- Gulick A. Phosphorus as a factor in the origin of life. Am. Sci. 1955;43:479–489.
-
- Gull M. Prebiotic phosphorylation reactions on the early Earth. Challenges. 2014;5:193–212. doi: 10.3390/challe5020193. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

