Treatment of Human Placental Choriocarcinoma Cells with Formaldehyde and Benzene Induced Growth and Epithelial Mesenchymal Transition via Induction of an Antioxidant Effect
- PMID: 28758930
- PMCID: PMC5580558
- DOI: 10.3390/ijerph14080854
Treatment of Human Placental Choriocarcinoma Cells with Formaldehyde and Benzene Induced Growth and Epithelial Mesenchymal Transition via Induction of an Antioxidant Effect
Abstract
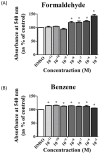
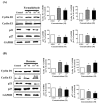
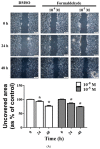
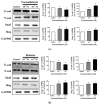
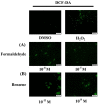
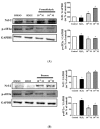
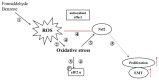
Cigarette smoke (CS) causes about 480,000 deaths each year worldwide, and it is well-known to have harmful effects on the human body, leading to heart disease, stroke, lung cancer, and cardiovascular problems. In this study, the effects of formaldehyde (FA) and benzene (Bz), the main components of CS, on cell proliferation and epithelial mesenchymal transition (EMT) of JEG-3 human choriocarcinoma cells were examined to confirm the relationship between CS components and placenta carcinoma. Upon MTT assay, FA (10-8 M to 10-5 M) and Bz (10-11 M to 10-8 M) increased JEG-3 cell proliferation. Western blot assay revealed that the protein expression of cyclin D1 and E1 increased, while the levels of p21 and p27 were reduced following treatment. In Scratch assay, FA (10-8 M and 10-5 M) and Bz (10-11 M and 10-8 M) increased migration of JEG-3 cells at 24 h and 48 h compared with that at 0 h. In addition, the expression of the epithelial marker, E-cadherin, was significantly decreased, while the expression of the mesenchymal marker, N-cadherin, was significantly increased by FA (10-8 M and 10-5 M) and Bz (10-11 M and 10-8 M). snail and slug transcriptional factors were associated with EMT, which were also up-regulated by FA and Bz, indicating that FA and Bz lead to an increase in the EMT process in JEG-3 choriocarcinoma cells. We further evaluated reactive oxygen species (ROS) and activation of antioxidant effect using dichlorofluorescin diacetate (DCFH-DA) and Western blot assay. FA and Bz increased the ROS production and an antioxidant related marker, Nrf2, in JEG-3 cells. However, eIF2α levels were reduced by FA and Bz via activation of the antioxidant reaction. Taken together, these results indicated that FA and Bz induce the growth and migration of human choriocarcinoma cells via regulation of the cell cycle and EMT and activation of ROS and antioxidant related markers.
Keywords: EMT; benzene; cell cycle; cigarette smoke; formaldehyde; placenta choriocarcinoma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Hanaki T., Horikoshi Y., Nakaso K., Nakasone M., Kitagawa Y., Amisaki M., Arai Y., Tokuyasu N., Sakamoto T., Honjo S., et al. Nicotine enhances the malignant potential of human pancreatic cancer cells via activation of atypical protein kinase C. Biochim. Biophys. Acta. 2016;1860(Pt A11):2404–2415. doi: 10.1016/j.bbagen.2016.07.008. - DOI - PubMed
-
- Kim C.W., Go R.E., Lee H.M., Hwang K.A., Lee K., Kim B., Lee M.Y., Choi K.C. Cigarette smoke extracts induced the colon cancer migration via regulating epithelial mesenchymal transition and metastatic genes in human colon cancer cells. Environ. Toxicol. 2016;32:690–704. doi: 10.1002/tox.22271. - DOI - PubMed
-
- Pesch B., Kendzia B., Gustavsson P., Jockel K.H., Johnen G., Pohlabeln H., Olsson A., Ahrens W., Gross I.M., Bruske I., et al. Cigarette smoking and lung cancer-relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer. 2012;131:1210–1219. doi: 10.1002/ijc.27339. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

