Ultrastructure and pathology of prion protein amyloid accumulation and cellular damage in extraneural tissues of scrapie-infected transgenic mice expressing anchorless prion protein
- PMID: 28759310
- PMCID: PMC5553302
- DOI: 10.1080/19336896.2017.1336274
Ultrastructure and pathology of prion protein amyloid accumulation and cellular damage in extraneural tissues of scrapie-infected transgenic mice expressing anchorless prion protein
Abstract
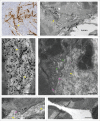
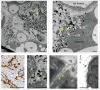
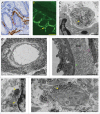
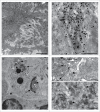
In most human and animal prion diseases the abnormal disease-associated prion protein (PrPSc) is deposited as non-amyloid aggregates in CNS, spleen and lymphoid organs. In contrast, in humans and transgenic mice with PrP mutations which cause expression of PrP lacking a glycosylphosphatidylinositol (GPI)-anchor, most PrPSc is in the amyloid form. In transgenic mice expressing only anchorless PrP (tg anchorless), PrPSc is deposited not only in CNS and lymphoid tissues, but also in extraneural tissues including heart, brown fat, white fat, and colon. In the present paper, we report ultrastructural studies of amyloid PrPSc deposition in extraneural tissues of scrapie-infected tg anchorless mice. Amyloid PrPSc fibrils identified by immunogold-labeling were visible at high magnification in interstitial regions and around blood vessels of heart, brown fat, white fat, colon, and lymphoid tissues. PrPSc amyloid was located on and outside the plasma membranes of adipocytes in brown fat and cardiomyocytes, and appeared to invaginate and disrupt the plasma membranes of these cell types, suggesting cellular damage. In contrast, no cellular damage was apparent near PrPSc associated with macrophages in lymphoid tissues and colon, with enteric neuronal ganglion cells in colon or with adipocytes in white fat. PrPSc localized in macrophage phagolysosomes lacked discernable fibrils and might be undergoing degradation. Furthermore, in contrast to wild-type mice expressing GPI-anchored PrP, in lymphoid tissues of tg anchorless mice, PrPSc was not associated with follicular dendritic cells (FDC), and FDC did not display typical prion-associated pathogenic changes.
Keywords: Alzheimer disease; CAA; amyloid; basement membrane; brown fat; cerebral amyloid angiopathy; colon; follicular dendritic cells; glycosaminoglycan; glycosylphosphatidylinositol; heart; lymphoid tissues; macrophages; prion protein; spleen; transmissible spongiform encephalopathies; white fat.
Figures


References
-
- Sipe JD, Benson MD, Buxbaum JN, Ikeda SI, Merlini G, Saraiva MJ, Westermark P. Amyloid fibril proteins and amyloidosis: Chemical identification and clinical classification International Society of Amyloidosis 2016 Nomenclature Guidelines. Amyloid 2016; 23:209-13; PMID:27884064; https://doi.org/10.1080/13506129.2016.1257986 - DOI - PubMed
-
- Pepys MB. Amyloidosis. Annu Rev Med 2006; 57:223-41; PMID:16409147; https://doi.org/10.1146/annurev.med.57.121304.131243 - DOI - PubMed
-
- Selkoe DJ. Alzheimer's disease: Genes, proteins, and therapy. Physiol Rev 2001; 81:741-66; PMID:11274343 - PubMed
-
- Bruce ME, Dickinson AG. Genetic control of amyloid plaque production and incubation period in scrapie-infected mice. J Neuropathol Exp Neurol 1985; 44:285-94; PMID:3921669; https://doi.org/10.1097/00005072-198505000-00006 - DOI - PubMed
-
- Jeffrey M, Goodsir CM, Holliman A, Higgins RJ, Bruce ME, McBride PA, Fraser JR. Determination of the frequency and distribution of vascular and parenchymal amyloid with polyclonal and N-terminal-specific PrP antibodies in scrapie-affected sheep and mice. Vet Rec 1998; 142:534-7; PMID:9637378; https://doi.org/10.1136/vr.142.20.534 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
