Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy
- PMID: 28759701
- PMCID: PMC6483146
- DOI: 10.1002/14651858.CD012744
Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy
Abstract
Background: Salivary gland dysfunction is an 'umbrella' term for the presence of either xerostomia (subjective sensation of dryness), or salivary gland hypofunction (reduction in saliva production). It is a predictable side effect of radiotherapy to the head and neck region, and is associated with a significant impairment of quality of life. A wide range of pharmacological interventions, with varying mechanisms of action, have been used for the prevention of radiation-induced salivary gland dysfunction.
Objectives: To assess the effects of pharmacological interventions for the prevention of radiation-induced salivary gland dysfunction.
Search methods: Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 14 September 2016); the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8) in the Cochrane Library (searched 14 September 2016); MEDLINE Ovid (1946 to 14 September 2016); Embase Ovid (1980 to 14 September 2016); CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 14 September 2016); LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 14 September 2016); Zetoc Conference Proceedings (1993 to 14 September 2016); and OpenGrey (1997 to 14 September 2016). We searched the US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the World Health Organization International Clinical Trials Registry Platform for ongoing trials. No restrictions were placed on the language or date of publication when searching the electronic databases.
Selection criteria: We included randomised controlled trials, irrespective of their language of publication or publication status. Trials included participants of all ages, ethnic origin and gender, scheduled to receive radiotherapy on its own or in addition to chemotherapy to the head and neck region. Participants could be outpatients or inpatients. We included trials comparing any pharmacological agent regimen, prescribed prophylactically for salivary gland dysfunction prior to or during radiotherapy, with placebo, no intervention or an alternative pharmacological intervention. Comparisons of radiation techniques were excluded.
Data collection and analysis: We used standard methodological procedures expected by Cochrane.
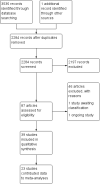
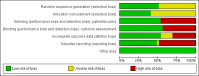
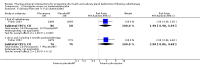
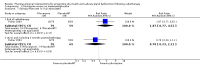
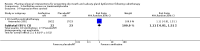
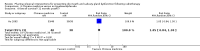
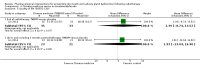
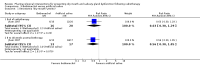
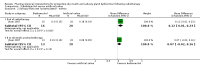
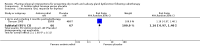
Main results: We included 39 studies that randomised 3520 participants; the number of participants analysed varied by outcome and time point. The studies were ordered into 14 separate comparisons with meta-analysis only being possible in three of those.We found low-quality evidence to show that amifostine, when compared to a placebo or no treatment control, might reduce the risk of moderate to severe xerostomia (grade 2 or higher on a 0 to 4 scale) at the end of radiotherapy (risk ratio (RR) 0.35, 95% confidence interval (CI) 0.19 to 0.67; P = 0.001, 3 studies, 119 participants), and up to three months after radiotherapy (RR 0.66, 95% CI 0.48 to 0.92; P = 0.01, 5 studies, 687 participants), but there is insufficient evidence that the effect is sustained up to 12 months after radiotherapy (RR 0.70, 95% CI 0.40 to 1.23; P = 0.21, 7 studies, 682 participants). We found very low-quality evidence that amifostine increased unstimulated salivary flow rate up to 12 months after radiotherapy, both in terms of mg of saliva per 5 minutes (mean difference (MD) 0.32, 95% CI 0.09 to 0.55; P = 0.006, 1 study, 27 participants), and incidence of producing greater than 0.1 g of saliva over 5 minutes (RR 1.45, 95% CI 1.13 to 1.86; P = 0.004, 1 study, 175 participants). However, there was insufficient evidence to show a difference when looking at stimulated salivary flow rates. There was insufficient (very low-quality) evidence to show that amifostine compromised the effects of cancer treatment when looking at survival measures. There was some very low-quality evidence of a small benefit for amifostine in terms of quality of life (10-point scale) at 12 months after radiotherapy (MD 0.70, 95% CI 0.20 to 1.20; P = 0.006, 1 study, 180 participants), but insufficient evidence at the end of and up to three months postradiotherapy. A further study showed no evidence of a difference at 6, 12, 18 and 24 months postradiotherapy. There was low-quality evidence that amifostine is associated with increases in: vomiting (RR 4.90, 95% CI 2.87 to 8.38; P < 0.00001, 5 studies, 601 participants); hypotension (RR 9.20, 95% CI 2.84 to 29.83; P = 0.0002, 3 studies, 376 participants); nausea (RR 2.60, 95% CI 1.81 to 3.74; P < 0.00001, 4 studies, 556 participants); and allergic response (RR 7.51, 95% CI 1.40 to 40.39; P = 0.02, 3 studies, 524 participants).We found insufficient evidence (that was of very low quality) to determine whether or not pilocarpine performed better or worse than a placebo or no treatment control for the outcomes: xerostomia, salivary flow rate, survival, and quality of life. There was some low-quality evidence that pilocarpine was associated with an increase in sweating (RR 2.98, 95% CI 1.43 to 6.22; P = 0.004, 5 studies, 389 participants).We found insufficient evidence to determine whether or not palifermin performed better or worse than placebo for: xerostomia (low quality); survival (moderate quality); and any adverse effects.There was also insufficient evidence to determine the effects of the following interventions: biperiden plus pilocarpine, Chinese medicines, bethanechol, artificial saliva, selenium, antiseptic mouthrinse, antimicrobial lozenge, polaprezinc, azulene rinse, and Venalot Depot (coumarin plus troxerutin).
Authors' conclusions: There is some low-quality evidence to suggest that amifostine prevents the feeling of dry mouth in people receiving radiotherapy to the head and neck (with or without chemotherapy) in the short- (end of radiotherapy) to medium-term (three months postradiotherapy). However, it is less clear whether or not this effect is sustained to 12 months postradiotherapy. The benefits of amifostine should be weighed against its high cost and side effects. There was insufficient evidence to show that any other intervention is beneficial.
Conflict of interest statement
Philip Riley: no interests to declare. Philip is an Editor with Cochrane Oral Health. Anne‐Marie Glenny: no interests to declare. Anne‐Marie is Deputy Co‐ordinating Editor of Cochrane Oral Health. Fang Hua: no interests to declare. Fang is an Editor with Cochrane Oral Health. Helen V Worthington: no interests to declare. Helen is Co‐ordinating Editor of Cochrane Oral Health.
Figures






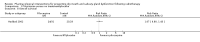






























Comment in
-
Insufficient evidence for interventions to prevent dry mouth and salivary gland dysfunction post head and neck radiotherapy.Evid Based Dent. 2018 Mar 23;19(1):30-31. doi: 10.1038/sj.ebd.6401295. Evid Based Dent. 2018. PMID: 29568026
References
References to studies included in this review
Abacioglu 1997 {unpublished data only}
-
- Abacioglu MU. Effect of Pilocarpine for the Prevention of Radiation‐Induced Xerostomia [Dissertation]. Marmara (Turkey): Marmara University Hospital, 1997.
Antonadou 2002 {published data only}
-
- Antonadou D, Pepelassi M, Synodinou M, Puglisi M, Throuvalas N. Prophylactic use of amifostine to prevent radiochemotherapy‐induced mucositis and xerostomia in head‐and‐neck cancer. International Journal of Radiation Oncology, Biology, Physics 2002;52(3):739‐47. - PubMed
Bardet 2011 {published data only}
-
- Bardet E, Martin L, Calais G, Alfonsi M, Feham N E, Tuchais C, et al. Subcutaneous compared with intravenous administration of amifostine in patients with head and neck cancer receiving radiotherapy: final results of the GORTEC2000‐02 phase III randomized trial. Journal of Clinical Oncology 2011;29(2):127‐33. - PubMed
-
- Bardet E, Martin L, Calais G, Tuchais C, Bourhis J, Rhein B, et al. Preliminary data of the GORTEC 2000‐02 phase III trial comparing intravenous and subcutaneous administration of amifostine for head and neck tumors treated by external radiotherapy. Seminars in Oncology 2002;29(6 Suppl 19):57‐60. - PubMed
Brizel 2000 {published data only}
-
- Brizel D, Sauer R, Wannenmacher M, Henke M, Eschwege F, Wasserman T. Randomized phase III trial of radiation amifostine in patients with head and neck cancer. 34th Annual Meeting of the American Society of Clinical Oncology; 1998 May 17‐19; Los Angeles (USA). Los Angeles (USA): American Society of Clinical Oncology, 1998. [Abs No 1487]
-
- Brizel DM, Wasserman T. The influence of intravenous amifostine on xerostomia and survival during radiotherapy for head and neck cancer: two year follow‐up of a prospective randomized trial. Journal of Clinical Oncology 2004;22(14 Suppl):5536. - PubMed
-
- Brizel DM, Wasserman TH, Henke M, Strnad V, Rudat V, Monnier A, et al. Phase III randomized trial of amifostine as a radioprotector in head and neck cancer. Journal of Clinical Oncology 2000;18(19):3339‐45. - PubMed
-
- Rudat V, Meyer J, Momm F, Bendel M, Henke M, Strnad V, et al. Protective effect of amifostine on dental health after radiotherapy of the head and neck. International Journal of Radiation Oncology, Biology, Physics 2000;48(5):1339‐43. - PubMed
-
- Strnad V, Sauer R. Radioprotection of head and neck tissue by amifostine. Frontiers of Radiation Therapy and Oncology 2002;37:101‐11. - PubMed
Brizel 2008 {published data only}
-
- Brizel DM, Murphy BA, Rosenthal DI, Pandya KJ, Glück S, Brizel HE, et al. Phase II study of palifermin and concurrent chemoradiation in head and neck squamous cell carcinoma. Journal of Clinical Oncology 2008;26(15):2489‐96. - PubMed
Buentzel 2006 {published data only}
-
- Buentzel J, Micke O, Adamietz IA, Monnier A, Glatzel M, Vries A. Intravenous amifostine during chemoradiotherapy for head‐and‐neck cancer: a randomized placebo‐controlled phase III study. International Journal of Radiation Oncology, Biology, Physics 2006;64(3):684‐91. - PubMed
Büntzel 1998 {published data only}
-
- Bennett CL, Lane D, Stinson T, Glatzel M, Buntzel J. Economic analysis of amifostine as adjunctive support for patients with advanced head and neck cancer: preliminary results from a randomized phase II clinical trial from Germany. Cancer Investigation 2001;19(2):107‐13. - PubMed
-
- Buntzel J, Glatzel M, Schuth J, Frohlich D, Kuttner K. Selective cytoprotection with amifostine: a new strategy in supportive care of head and neck malignancies. Oto‐Rhino‐Laryngologia Nova 1997;7:276‐80.
-
- Buntzel J, Kuttner K, Russell L, Oster W, Schuth J, Glatzel M. Selective cytoprotection by amifostine (A) in the treatment of head and neck cancer with simultaneous radiochemotherapy (RCT). European Cancer Conference; 1997 Sep 14‐18; Hamburg (Germany). Hamburg (Germany): ECCO, 1997. [Abs No 852]
-
- Buntzel J, Kuttner K, Russell L, Oster W, Schuth J, Glatzel M. Selective cytoprotection by amifostine in the treatment of head and neck cancer with simultaneous radiochemotherapy. Proceedings of the American Society of Clinical Oncology 1997;16:393. [Abs No 1400]
-
- Büntzel J. Experiences with selenium in the treatment of acute and late toxicities due to radiochemotherapy of head and neck cancer [Erfahrungen mit Natriumselenit in der Behandlung von akuten und späten Nebenwirkungen der Radiochemotherapie von Kopf‐Hals‐Karzinomen]. Medizinische Klinik 1999;94 Suppl III:49‐53. - PubMed
Büntzel 2010 {published data only}
-
- Buentzel J, Micke O, Glatzel M, Bruns F, Kisters K, Muecke R. Evaluation of the effect of selenium on radiation‐induced toxicities in head neck cancer patients. Journal of Clinical Oncology 2009;27(15 Suppl):e20698.
-
- Büntzel J, Micke O, Glatzel M, Schäfer U, Riesenbeck D, Kisters K, et al. Selenium substitution during radiotherapy in head and neck cancer. Trace Elements and Electrolytes 2010;27:235‐9.
-
- Büntzel J, Riesenbeck D, Glatzel M, Berndt‐Skorka R, Riedel T, Mücke R, et al. Limited effects of selenium substitution in the prevention of radiation‐associated toxicities. Results of a randomized study in head and neck cancer patients. Anticancer Research 2010;30(5):1829‐32. - PubMed
Burlage 2008 {published data only}
-
- Burlage FR, Roesink JM, Kampinga HH, Coppes RP, Terhaard C, Langendijk JA, et al. Protection of salivary function by concomitant pilocarpine during radiotherapy: a double‐blind, randomized, placebo‐controlled study. International Journal of Radiation Oncology, Biology, Physics 2008;70(1):14‐22. - PubMed
Duncan 2005 {published data only}
-
- Duncan GG, Epstein JB, Tu D, Sayed S, Bezjak A, Ottaway J, et al. Quality of life, mucositis, and xerostomia from radiotherapy for head and neck cancers: a report from the NCIC CTG HN2 randomized trial of an antimicrobial lozenge to prevent mucositis. Head & Neck 2005;27(5):421‐8. - PubMed
Fisher 2003 {published data only}
-
- Fisher J, Scott C, Scarantino CW, Leveque FG, White RL, Rotman M, et al. Phase III quality‐of‐life study results: impact on patients' quality of life to reducing xerostomia after radiotherapy for head‐and‐neck cancer ‐ RTOG 97‐09. International Journal of Radiation Oncology, Biology, Physics 2003;56(3):832‐6. - PubMed
-
- Scarantino C, LeVeque F, Swann RS, White R, Schulsinger A, Hodson I, et al. Effect of pilocarpine during radiation therapy: results of RTOG 97‐09, a phase III randomized study in head and neck cancer patients. Journal of Supportive Oncology 2006;4(5):252‐8. - PubMed
-
- Scarantino CW, Leveque F, Scott C, White RL, Rotman M, Hodson DI, et al. A phase III study on the concurrent use of oral pilocarpine to reduce hyposalivation and mucositis associated with radiation therapy in head and neck cancer patients. Final results of RTOG 97‐09. International Journal of Radiation Oncology, Biology, Physics 2001;51(3 Suppl 1):85‐6. [Abs No 157]
-
- Scarantino CW, Leveque FG, Scott CB, White RL, Rotman M, Hodson DI. A phase III study of concomitant oral pilocarpine to reduce hypo‐salivation and mucositis associated with curative radiation therapy (RT) in head and neck (H&N) cancer patients. RTOG 9709. Proceedings of the American Society of Clinical Oncology 2001;20:225a. [Abs No 897]
Gornitsky 2004 {published and unpublished data}
-
- Gornitsky M, Shenouda G, Sultanem K, Katz H, Hier M, Black M, et al. Double‐blind randomized, placebo‐controlled study of pilocarpine to salvage salivary gland function during radiotherapy of patients with head and neck cancer. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2004;98(1):45‐52. - PubMed
Grötz 2001 {published data only}
-
- Grötz KA, Henneicke‐Von Zepelin HH, Kohnen R, Kutzner J, Belz GG. Prophylaxis of mucositis and dry mouth after head and neck radiotherapy. A new treatment. Krankenhauspharmazie 2002;23(5):193‐8.
-
- Grötz KA, Henneicke‐von Zepelin HH, Kohnen R, al‐Nawas B, Bockisch A, Kutzner J, et al. Prospective double‐blind study of prophylaxis of radioxerostomia with Coumarin/Troxerutine in patients with head and neck cancer [Prospektive, doppelblinde Therapiestudie zur Prophylaxe der Radioxerostomie durch Cumarin/Troxerutin bei Patienten mit Kopf‐Hals‐Karzinomen]. Strahlentherapie und Onkologie 1999;175(8):397‐404. - PubMed
-
- Grötz KA, Wüstenberg P, Kohnen R, Al‐Nawas B, Henneicke‐von Zepelin H, Bockisch A, et al. Prophylaxis of radiogenic sialadenitis and mucositis by coumarin/troxerutine in patients with head and neck cancer ‐ a prospective, randomized, placebo‐controlled, double‐blind study. British Journal of Oral and Maxillofacial Surgery 2001;39(1):34‐9. - PubMed
Haddad 2002 {published data only}
-
- Haddad P, Karimi M. A randomised, double‐blind, placebo‐controlled trial of concomitant pilocarpine with head and neck irradiation for prevention of radiation‐induced xerostomia. Radiotherapy and Oncology 2002;64(1):29‐32. - PubMed
Haddad 2009 {published data only}
-
- Haddad R, Sonis S, Posner M, Wirth L, Costello R, Braschayko P, et al. Randomized phase 2 study of concomitant chemoradiotherapy using weekly carboplatin/paclitaxel with or without daily subcutaneous amifostine in patients with locally advanced head and neck cancer. Cancer 2009;115(19):4514‐23. - PubMed
Han 2010 {published data only}
-
- Han F, Zhang JD, Shao ZY, Liu FJ. Effects of concurrent chemoradiotherapy combined with Jinlong capsules for patients with advanced nasopharyngeal squamous carcinoma. Chinese Journal of Cancer Prevention and Treatment 2010;(2):138‐9.
He 2004 {published data only}
-
- He X, Hu C, Wu Y. Radioprotective effect of amifostine in nasopharyngeal carcinoma. Fudan University Journal of Medical Sciences 2004;31(1):53‐8.
Henke 2011 {published data only}
-
- Henke M, Alfonsi M, Foa P, Giralt J, Bardet E, Cerezo L, et al. Palifermin decreases severe oral mucositis of patients undergoing postoperative radiochemotherapy for head and neck cancer: a randomized, placebo‐controlled trial. Journal of Clinical Oncology 2011;29(20):2815‐20. - PubMed
Hu 2005 {published data only}
-
- Hu YR, Wu CQ, Liu YJ, Wang Y, Li X, Zhong H, et al. Clinical observation on effect of shenqi fanghou recipe in preventing and treating radiation injury in patients with head and neck tumour. Zhongguo Zhong Xi Yi Jie He Za Zhi 2005;25(7):623‐5. - PubMed
Jaguar 2015 {published data only}
-
- Jaguar GC. A Prospective Study of Bethanechol in the Salivary Glands Physiology in Patients Following Head and Neck Radiotherapy [Estudo Prospectivo do Uso do Betanecol na Fisiologia de Glândulas Salivares em Pacientes Irradiados em Região de Cabeça e Pescoço] [Dissertation]. Sao Paulo (Brazil): Fundaçao Antonio Prudente, 2010.
-
- Jaguar GC, Campos L, Pellizzon AC, Lima EN, Kowalski LP, Alves FA. Double‐blind randomized prospective trial of bethanechol in the prevention of radiation‐induced salivary gland dysfunction in cancer patients. Supportive Care in Cancer 2012;20(1 Suppl):S252. - PubMed
-
- Jaguar GC, Lima EN, Kowalski LP, Pellizzon AC, Carvalho AL, Boccaletti KW, et al. Double blind randomized prospective trial of bethanechol in the prevention of radiation‐induced salivary gland dysfunction in head and neck cancer patients. Radiotherapy and Oncology 2015;115(2):253‐6. - PubMed
Jellema 2006 {published data only}
-
- Jellema AP, Slotman BJ, Muller MJ, Leemans CR, Smeele LE, Hoekman K, et al. Radiotherapy alone, versus radiotherapy with amifostine 3 times weekly, versus radiotherapy with amifostine 5 times weekly: a prospective randomized study in squamous cell head and neck cancer. Cancer 2006;107(3):544‐53. - PubMed
Jham 2007 {published data only}
-
- Jham BC, Chen H, Carvalho AL, Freire AR. A randomized phase III prospective trial of bethanechol to prevent mucositis, candidiasis, and taste loss in patients with head and neck cancer undergoing radiotherapy: a secondary analysis. Journal of Oral Science 2009;51(4):565‐72. - PubMed
-
- Jham BC, Teixeira IV, Aboud CG, Carvalho AL, Coelho Mde M, Freire AR. A randomized phase III prospective trial of bethanechol to prevent radiotherapy‐induced salivary gland damage in patients with head and neck cancer. Oral Oncology 2007;43(2):137‐42. - PubMed
Lajtman 2000 {published data only}
-
- Lajtman Z, Krajina Z, Krpan D, Vincelj J, Borcic V, Popovic‐Kovacic J. Pilocarpine in the prevention of postirradiation xerostomia. Acta Medica Croatica 2000;54(2):65‐7. - PubMed
Lanzós 2010 {published data only}
-
- Lanzós I, Herrera D, Santos S, O'Connor A, Peña C, Lanzós E, et al. Mucositis in irradiated cancer patients: effects of an antiseptic mouthrinse. Medicina Oral, Patología Oral y Cirugía Bucal 2010;15(5):e732‐8. - PubMed
Le 2011 {published data only}
-
- Le QT, Kim HE, Schneider CJ, Murakozy G, Skladowski K, Reinisch S, et al. Palifermin reduces severe mucositis in definitive chemoradiotherapy of locally advanced head and neck cancer: a randomized, placebo‐controlled study. Journal of Clinical Oncology 2011;29(20):2808‐14. - PubMed
Lin 2014 {published data only}
-
- Lin KS, Jen YM, Chao TY, Lin YS, Wang LH, Lin CJ, et al. Prevention of acute radiation‐associated toxicity by traditional chinese medicine Tianwang Buxin mini‐pills in patients with head and neck cancer. Journal of Medical Sciences (Taipei, Taiwan) 2014;34:152‐60.
Lozada‐Nur 1998 {published and unpublished data}
-
- Lozada‐Nur F, Schoelch M, Fu K, Muscoplat C, Trivedi M, Smith C, et al. A pilot study to evaluate the effect of pilocarpine tablets on salivary flow and mucositis in head and neck cancer patients during radiotherapy. Proceedings of the American Society of Clinical Oncology 1998;17:399. [Abs No 1541]
Patni 2004 {published and unpublished data}
-
- Patni N, Patni S, Bapna A, Somani N, Gupta A, Ratnam BV. The role of amifostine in prophylaxis of radiotherapy induced mucositis and xerostomia in head and neck cancer. Journal of Clinical Oncology 2004;22(14 Suppl):5568.
Peng 2006 {published data only}
-
- Peng LH, Sun Y, Zhang XL, Zhang Q, Fu S. Efficacy of amifostine on patients with head and neck squamous cell carcinomas undergoing radiation therapy and chemotherapy and clinical observation on its hematologic toxicity. Journal of China Pharmacy 2006;17(5):1166‐8.
Pimentel 2014 {published data only}
-
- Pimentel MJ, Filho MM, Araújo M, Gomes DQ, Costa LJ. Evaluation of radioprotective effect of pilocarpine ingestion on salivary glands. Anticancer Research 2014;34(4):1993‐9. - PubMed
Reshma 2012 {published data only}
-
- Reshma K, Kamalaksh S, Bindu YS, Pramod K, Asfa A, Amritha D, et al. Tulasi (Ocimum Sanctum) as radioprotector in head and neck cancer patients undergoing radiation therapy. Biomedicine 2012;32:39‐44.
Rode 1999 {published data only}
-
- Rode M, Smid L, Budihna M, Soba E, Rode M, Gaspersic D. The effect of pilocarpine and biperiden on salivary secretion during and after radiotherapy in head and neck cancer patients. International Journal of Radiation Oncology, Biology, Physics 1999;45(2):373‐8. - PubMed
Sangthawan 2001 {published data only}
-
- Sangthawan D, Watthanaarpornchai S, Phungrassami T. Randomized double blind, placebo‐controlled study of pilocarpine administered during head and neck irradiation to reduce xerostomia. Journal of the Medical Association of Thailand 2001;84(2):195‐203. - PubMed
-
- Sangthawan D, Watthanaarpornchai S, Phungrassami T. Randomized double blind, placebo‐controlled study of pilocarpine administered during head and neck irradiation to reduce xerostomia. Radiation Oncology Association of Thailand 2002;7:242. - PubMed
Vacha 2003 {published data only}
-
- Vacha P, Fehlauer F, Mahlmann B, Marx M, Hinke A, Sommer K, et al. Randomized phase III trial of postoperative radiochemotherapy +/‐ amifostine in head and neck cancer. Is there evidence for radioprotection?. Strahlentherapie und Onkologie 2003;179(6):385‐9. - PubMed
-
- Vacha P, Marx M, Engel A, Richter E, Feyerabend T. Side effects of postoperative radiochemotherapy with amifostine versus radiochemotherapy alone in head and neck tumors. Preliminary results of a prospective randomized trial. Strahlentherapie und Onkologie 1999;175 Suppl 4:18‐22. - PubMed
Valdez 1993 {published data only}
-
- Valdez IH, Wolff A, Atkinson JC, Macynski AA, Fox PC. Use of pilocarpine during head and neck radiation therapy to reduce xerostomia and salivary dysfunction. Cancer 1993;71(5):1848‐51. - PubMed
-
- Wolff A, Atkinson JC, Macynski AA, Fox PC. Oral complications of cancer therapies. Pretherapy interventions to modify salivary dysfunction. NCI Monographs 1990;(9):87‐90. - PubMed
Veerasarn 2006 {published and unpublished data}
-
- Veerasarn V, Phromratanapongse P, Suntornpong N, Lorvidhaya V, Chitapanarux I, Tesavibul C, et al. Effect of amifostine to prevent radiotherapy‐induced acute and late toxicity in head and neck cancer patients who had normal or mild impaired salivary gland dysfunction. European Journal of Cancer Supplements 2003;1(5):S273. [Abs No 908] - PubMed
-
- Veerasarn V, Phromratanapongse P, Suntornpong N, Lorvidhaya V, Sukthomya V, Chitapanarux I, et al. Effect of amifostine to prevent radiotherapy‐induced acute and late toxicity in head and neck cancer patients who had normal or mild impaired salivary gland function. Journal of the Medical Association of Thailand 2006;89(12):2056‐67. - PubMed
Wang 1998 {published data only}
-
- Wang Q, Liu H, Qiao N. Effects of traditional Chinese medicine on salivary glands in the patients with head and neck cancer during radiotherapy. Zhongguo Zhong Xi Yi Jie He Za Zhi 1998;18(11):662‐4. - PubMed
Warde 2002 {published data only}
-
- Warde P, O'Sullivan B, Aslanidis J, Kroll B, Lockwood G, Math M, et al. A phase III placebo‐controlled trial of oral pilocarpine in patients undergoing radiotherapy for head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 2002;54(1):9‐13. - PubMed
Watanabe 2010 {published data only}
-
- Watanabe T, Ishihara M, Matsuura K, Mizuta K, Itoh Y. Polaprezinc prevents oral mucositis associated with radiochemotherapy in patients with head and neck cancer. International Journal of Cancer 2010;127(8):1984‐90. - PubMed
References to studies excluded from this review
Anné 2002 {published data only}
-
- Anné PR. Phase II trial of subcutaneous amifostine in patients undergoing radiation therapy for head and neck cancer. Seminars in Oncology 2002;29(6 Suppl 19):80‐3. - PubMed
Bagga 2007 {published data only}
-
- Bagga P, Anand AK, Raina A, Choudhary PS, Chaudhoory AR. Evaluation of pilocarpine for the prevention of radiation induced xerostomia in head and neck cancers. Annals of Oncology 2007;18(Suppl 9):ix177. [Abs No 62]
Bakowski 1978 {published data only}
-
- Bakowski MT, Macdonald E, Mould RF, Cawte P, Sloggem J, Barrett A, et al. Double blind controlled clinical trial of radiation plus razoxane (ICRF 159) versus radiation plus placebo in the treatment of head and neck cancer. International Journal of Radiation Oncology, Biology, Physics 1978;4(1‐2):115‐9. - PubMed
Belcaro 2008 {published data only}
-
- Belcaro G, Cesarone MR, Genovesi D, Ledda A, Vinciguerra G, Ricci A, et al. Pycnogenol may alleviate adverse effects in oncologic treatment. Panminerva Medica 2008;50(3):227‐34. - PubMed
Bohuslavizki 1998 {published data only}
-
- Bohuslavizki KH, Klutmann S, Bleckmann C, Brenner W, Lassmann S, Mester J, et al. Salivary gland protection by amifostine in high‐dose radioiodine therapy of differentiated thyroid cancer. Strahlentherapie und Onkologie 1999;175(2):57‐61. - PubMed
-
- Bohuslavizki KH, Klutmann S, Brenner W, Kröger S, Buchert R, Bleckmann C, et al. Radioprotection of salivary glands by amifostine in high‐dose radioiodine treatment. Results of a double‐blinded, placebo‐controlled study in patients with differentiated thyroid cancer. Strahlentherapie und Onkologie 1999;175(Suppl 4):6‐12. - PubMed
-
- Bohuslavizki KH, Klutmann S, Brenner W, Mester J, Henze E, Clausen M. Salivary gland protection by amifostine in high‐dose radioiodine treatment: results of a double‐blind placebo‐controlled study. Journal of Clinical Oncology 1998;16(11):3542‐9. - PubMed
-
- Bohuslavizki KH, Klutmann S, Jenicke L, Kröger S, Buchert R, Mester J, et al. Salivary gland protection by S‐2‐(3‐amino‐propylamino)‐ethylphosphorothioic acid (amifostine) in high‐dose radioiodine treatment: results obtained in a rabbit animal model and in a double‐blind multi‐arm trial. Cancer Biotherapy & Radiopharmaceuticals 1999;14(5):337‐47. - PubMed
Borg 2007 {published data only}
-
- Borg M, Krishnan S, Olver L, Stein B, Chatterton B, Coates E, et al. Randomised double‐blind trial of amifostine vs placebo for radiation‐induced xerostomia in patients with head and neck cancer. ANZ Journal of Surgery 2007;77(12):A3.
Bourhis 2000 {published data only}
-
- Bourhis J, Crevoisier R, Abdulkarim B, Deutsch E, Lusinchi A, Luboinski B, et al. A randomized study of very accelerated radiotherapy with and without amifostine in head and neck squamous cell carcinoma. International Journal of Radiation Oncology, Biology, Physics 2000;46(5):1105‐8. - PubMed
Braaksma 2002 {published data only}
-
- Braaksma M, Levendag P. Tools for optimal tissue sparing in concomitant chemoradiation of advanced head and neck cancer: subcutaneous amifostine and computed tomography‐based target delineation. Seminars in Oncology 2002;29(6 Suppl 19):63‐70. - PubMed
Braaksma 2005 {published data only (unpublished sought but not used)}
-
- Braaksma M, Agthoven M, Nijdam W, Uyl‐de Groot C, Levendag P. Costs of treatment intensification for head and neck cancer: concomitant chemoradiation randomised for radioprotection with amifostine. European Journal of Cancer 2005;41(14):2102‐11. - PubMed
Chambers 2005 {published data only}
-
- Chambers MS, Posner MR, Jones CU, Weber RS, Vitti R. Two phase III clinical studies of cevimeline for post‐radiation xerostomia in patients with head and neck cancer. Journal of Clinical Oncology 2005;23(16 Suppl):5503.
Demiroz 2012 {published data only}
-
- Demiroz C, Ozcan L, Cebelli G, Karadag O, Ozsahin EM. The effect of amifostine on acute and late radiation side effects in head and neck cancer patients. Turkiye Klinikleri Journal of Medical Sciences 2012;32:1207‐16.
Fallahi 2013 {published data only}
-
- Fallahi B, Beiki D, Abedi SM, Saghari M, Fard‐Esfahani A, Akhzari F, et al. Does vitamin E protect salivary glands from I‐131 radiation damage in patients with thyroid cancer?. Nuclear Medicine Communications 2013;34(8):777‐86. - PubMed
Fan 2011 {published data only}
-
- Fan Z, Lin H, Liu Z, He Q, Zhang S, Li W. Protective effect of amifostine in irradiation mucositis of the oral cavity and dry mouth after nasapharyngeal carcinoma. Military Medical Journal of Southeast China 2011;13(2):146‐8.
Franzén 1995 {published data only}
-
- Franzén L, Henriksson R, Littbrand B, Zackrisson B. Effects of sucralfate on mucositis during and following radiotherapy of malignancies in the head and neck region. A double‐blind placebo‐controlled study. Acta Oncologica (Stockholm, Sweden) 1995;34(2):219‐23. - PubMed
Fuertes 2004 {published data only}
-
- Fuertes Cabero S, Setoain Perego X, Rovirosa Casino A, Mateos Fernández JJ, Fuster Pelfort D, Ferre Jorge J, et al. Usefulness of pilocarpine in the prevention of xerostomia in patients with head and neck cancer treated with radiotherapy. Assessment with gammagraphy and salivary flow [Utilidad de la pilocarpina como profiláctico de xerostomia en pacientes con cáncer de cabeza y cuello tratados con radioterapia. Valoración mediante gammagrafía y flujo salivar]. Revista Española de Medicina Nuclear 2004;23(4):259‐66. - PubMed
Goyal 2007 {published data only}
-
- Goyal S, Sharma DN, Julka PK, Rath GK. Effect of oral pilocarpine on xerostomia and quality of life in patients receiving curative radiotherapy for head and neck cancers. Journal of Clinical Oncology 2007;25(18 Suppl):6088.
Gu 2014 {published data only}
Johnson 2002 {published data only}
-
- Johnson DJ, Scott CB, Marks JE, Seay TE, Atkins JN, Berk LB, et al. Assessment of quality of life and oral function of patients participating in a phase II study of radioprotection of oral and pharyngeal mucosa by the prostaglandin E(1) analog misoprostol (RTOG 96‐07). International Journal of Radiation Oncology, Biology, Physics 2002;54(5):1455‐9. - PubMed
Karacetin 2004 {published data only}
-
- Karacetin D, Yücel B, Leblebicioglu B, Aksakal O, Maral O, Incekara O. A randomized trial of amifostine as radioprotector in the radiotherapy of head and neck cancer. Journal of BUON 2004;9(1):23‐6. - PubMed
Koukourakis 2000 {published data only}
-
- Koukourakis MI, Kyrias G, Kakolyris S, Kouroussis C, Frangiadaki C, Giatromanolaki A, et al. Subcutaneous administration of amifostine during fractionated radiotherapy: a randomized phase II study. Journal of Clinical Oncology 2000;18(11):2226‐33. - PubMed
Kumarchandra 2010 {published data only}
-
- Kumarchandra R, Shenoy K, Bindu YS, Kadaba P, Chandrashekar R. Ocimum Sanctum as a radioprotector in head and neck cancer patients undergoing radiation therapy. Free Radical Biology and Medicine 2010;49 Suppl:S64.
Manoor 2014 {published data only}
-
- Manoor Maiya V, Vaid N, Basu S, Vatyam S, Hegde S, Deshmukh S, et al. The use of xylitol for the prevention of xerostomia in patients receiving intensity modulated radiation therapy for head and neck cancers. International Journal of Radiation Oncology, Biology, Physics 2014;90(1 Suppl):S562.
Mateos 2001 {published data only}
-
- Mateos JJ, Setoain X, Ferre J, Rovirosa A, Navalpotro B, Martin F, et al. Salivary scintigraphy for assessing the protective effect of pilocarpine in head and neck irradiated tumours. Nuclear Medicine Communications 2001;22(6):651‐6. - PubMed
Mitine 2000 {published data only}
-
- Mitine C, Chaltin M, Salembier C, Merlo P, Beauduin M. Head and neck radiotherapy: does pilocarpine hydrochloride reduce radiation‐induced xerostomia?. Proceedings of the management of head and neck tumors: what are the challenges for the third millennium; 1999 Dec 3‐4; Brussels (Belgium). European Laryngological Society, 1999.
Mix 2013 {published data only}
-
- Mix MD, Jameson M, Tills M, Dibaj S, Groman A, Jaggernauth W, et al. Randomized double‐blind, placebo‐controlled, multicenter phase 2 trial of selenomethionine as a modulator of efficacy and toxicity of chemoradiation in locally‐advanced squamous cell carcinoma of the head and neck. International Journal of Radiation Oncology, Biology, Physics 2013;87(2 Suppl):S466‐7.
Nicolatou‐Galitis 2003 {published data only}
-
- Nicolatou‐Galitis O, Sotiropoulou‐Lontou A, Velegraki A, Pissakas G, Kolitsi G, Kyprianou K, et al. Oral candidiasis in head and neck cancer patients receiving radiotherapy with amifostine cytoprotection. Oral Oncology 2003;39:397‐401. - PubMed
Norberg‐Spaak 1996 {published data only}
-
- Norberg‐Spaak LE, Lundquist PG, Berndtson M, Klintenberg C. Can pilocarpine treatment during irradiation prevent salivary gland damage?. Swedish Dental Journal 1996;20:234.
Norberg‐Spaak 1997 {published data only}
-
- Norberg‐Spaak LE. Can pilocarpine treatment during irradiation prevent salivary gland damage. Clinical Otolaryngology and Allied Sciences 1997;22:88.
Nyárády 2006 {unpublished data only}
-
- Nyárády Z, Németh A, Bán A, Mukics A, Nyárády J, Ember I, et al. A randomized study to assess the effectiveness of orally administered pilocarpine during and after radiotherapy of head and neck cancer. Anticancer Research 2006;26(2B):1557‐62. - PubMed
-
- Nyárády Z, Németh Á, Mukics A, Olasz L. Relieving symptoms of xerostomia with oral pilocarpine (Salagen) during irradiation in head‐and‐neck cancer. Journal of Cranio‐Maxillo‐Facial Surgery 2004;32 Suppl 1:230. [Abs No 459]
Park 2012 {published data only}
-
- Park J, McGuire DB, Kang H. Effects of cold sterile normal saline (CSNS) mouth care in head and neck cancer (HNC) patients undergoing concurrent chemoradiotherapy (CCRT). Supportive Care in Cancer 2012;20(1 Suppl):S246‐7. [Abs No 1028]
Park 2012a {published data only}
-
- Park K‐N, Son Y‐I, Baek CH. Protection of radiotherapy‐induced xerostomia with vitamin E+C complex. Otolaryngology ‐ Head and Neck Surgery 2012;147(2 Suppl):P174.
Peters 1999 {published data only}
-
- Peters K, Mücke R, Hamann D, Ziegler PG, Fietkau R. Supportive use of amifostine in patients with head and neck tumours undergoing radio‐chemotherapy. Is it possible to limit the duration of the application of amifostine?. Strahlentherapie und Onkologie 1999;175 Suppl 4:23‐6. - PubMed
Qian 2003 {published data only}
-
- Qian Y. Effects of Traditional Chinese Medicine on Salivary Glands of Patients with Head and Neck Cancer During Radiotherapy [Dissertation]. China National Knowledge Infrastructure (CNKI), 2003.
Resubal 2011 {published data only}
-
- Resubal JR, Calaguas MJ. The effect of zilongjin(r) in patients with head and neck cancer needing radiotherapy with and without chemotherapy. Radiotherapy and Oncology 2011;99(Suppl 1):S336.
Rieger 2012 {published data only}
-
- Rieger JM, Jha N, Lam Tang JA, Harris J, Seikaly H. Functional outcomes related to the prevention of radiation‐induced xerostomia: oral pilocarpine versus submandibular salivary gland transfer. Head & Neck 2012;34(2):168‐74. - PubMed
Rischin 2010 {published data only}
-
- Rischin D, Peters LJ, O'Sullivan B, Giralt J, Fisher R, Yuen K, et al. Tirapazamine, cisplatin, and radiation versus cisplatin and radiation for advanced squamous cell carcinoma of the head and neck (TROG 02.02, HeadSTART): a phase III trial of the Trans‐Tasman Radiation Oncology Group. Journal of Clinical Oncology 2010;28(18):2989‐95. - PubMed
Rudat 2005 {published data only}
-
- Rudat V, Münter M, Rades D, Grötz K, Haberkorn U, Brenner W. The effect of amifostine or IMRT to preserve the parotid function after radiotherapy of the head and neck region measured by quantitative salivary gland scintigraphy. Journal of Clinical Oncology 2005;23(16 Suppl):5502. - PubMed
Schönekäs 1999 {published data only}
-
- Schönekäs KG, Wagner W, Prott FJ. Amifostine ‐ a radioprotector in locally advanced head and neck tumours. Strahlentherapie und Onkologie 1999;175 Suppl 4:27‐9. - PubMed
Sharma 2012 {published data only}
-
- Sharma A, Rath GK, Chaudhary SP, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation‐ and chemotherapy‐induced mucositis in patients with head and neck cancer: a randomized double‐blind placebo‐controlled study. European Journal of Cancer 2012;48(6):875‐81. - PubMed
Strnad 1997 {published data only}
-
- Strnad V, Sauer R, Krafft T, Lerch S. Preliminary results of a randomised study using WR‐2721 in radiation therapy alone in patients with head and neck cancer. European Journal of Cancer 1997;33(Suppl 8):S189.
Su 2006 {published data only}
-
- Su YB, Vickers AJ, Zelefsky MJ, Kraus DH, Shaha AR, Shah JP, et al. Double‐blind, placebo‐controlled, randomized trial of granulocyte‐colony stimulating factor during postoperative radiotherapy for squamous head and neck cancer. Cancer Journal (Sudbury, Mass.) 2006;12(3):182‐8. - PubMed
Takahashi 1986 {published data only}
-
- Takahashi I, Nagai T, Miyaishi K, Maehara Y, Niibe H. Clinical study of the radioprotective effects of amifostine (YM‐08310, WR‐2721) on chronic radiation injury. International Journal of Radiation Oncology, Biology, Physics 1986;12(6):935‐8. - PubMed
Thorstad 2003 {published data only}
-
- Thorstad WL, Haughey B, Chao KS. Pilot study of subcutaneous amifostine in patients undergoing postoperative intensity modulated radiation therapy for head and neck cancer: preliminary data. Seminars in Oncology 2003;30(6 Suppl 18):96‐100. - PubMed
Uchiyama 2005 {published data only}
-
- Uchiyama Y, Murakami S, Kakimoto N, Nakatani A, Furukawa S. Effectiveness of cepharanthin in decreasing interruptions during radiation therapy for oral cancer. Oral Radiology 2005;21(1):41‐4.
Zale 1993 {published data only}
-
- Zale M, Chambers MS, Khan Z. Pilocarpine effects on xerostomia associated with radiation therapy. Journal of Dental Research 1993;72(Abs Spec Issue):375. [Abs No 2175]
Zimmerman 1997 {published data only}
-
- Zimmerman RP, Mark RJ, Juillard GF. Timing of pilocarpine treatment during head and neck radiotherapy: concomitant administration reduces xerostomia better than post‐radiation pilocarpine. International Journal of Radiation Oncology, Biology, Physics 1996;36 Suppl 1(1):236. [Abs No 155]
-
- Zimmerman RP, Mark RJ, Tran LM, Juillard GF. Concomitant pilocarpine during head and neck irradiation is associated with decreased posttreatment xerostomia. International Journal of Radiation Oncology, Biology, Physics 1997;37(3):571‐5. - PubMed
-
- Zimmerman RP, Rufus JM, Juillard GF. Concomitant pilocarpine during head and neck irradiation reduces xerostomia. Supportive Care in Cancer 1996;4(3):243. [Abs No 76]
References to studies awaiting assessment
Yu 2009 {published data only}
-
- Yu DS, Zhao W, Wu XL, Huang ZY, Huang RB. Amifostine in prevention of radiation‐induced xerostomia. Journal of Sun Yat‐Sen University (Medical Sciences) 2009;30(4S):86‐7, 95.
References to ongoing studies
NCT02430298 {published data only}
-
- NCT02430298. Topical/oral melatonin for preventing concurrent radiochemotherapy induced oral mucositis/xerostomia cancer patients [Topical and oral melatonin for preventing concurrent radiochemotherapy induced oral mucositis and xerostomia in head and neck cancer patients]. clinicaltrials.gov/show/NCT02430298 (first received 28 January 2014).
Additional references
Ahlner 1994
-
- Ahlner BH, Hagelqvist E, Lind MG. Influence on rabbit submandibular gland injury by stimulation or inhibition of gland function during irradiation. Histology and morphometry after 15 gray. Annals of Otology, Rhinology, and Laryngology 1994;103(2):125‐34. - PubMed
Andreassen 2003
-
- Andreassen CN, Grau C, Lindegaard JC. Chemical radioprotection: a critical review of amifostine as a cytoprotector in radiotherapy. Seminars in Radiation Oncology 2003;13(1):62‐72. - PubMed
Bennett 2001
-
- Bennett CL, Lane D, Stinson T, Glatzel M, Buntzel J. Economic analysis of amifostine as adjunctive support for patients with advanced head and neck cancer: preliminary results from a randomized phase II clinical trial from Germany. Cancer Investigations 2001;19(2):107‐13. - PubMed
Bohuslavizki 1998
-
- Bohuslavizki KH, Brenner W, Klutmann S, Hübner RH, Lassmann S, Feyerabend B, et al. Radioprotection of salivary glands by amifostine in high‐dose radioiodine therapy. Journal of Nuclear Medicine 1998;39(7):1237‐42. - PubMed
Buglione 2016
-
- Buglione M, Cavagnini R, Rosario F, Maddalo M, Vassalli L, Grisanti S, et al. Oral toxicity management in head and neck cancer patients treated with chemotherapy and radiation: Xerostomia and trismus (Part 2). Literature review and consensus statement. Critical Reviews in Oncology/Hematology 2016;102:47‐54. - PubMed
Clarkson 2010
Davies 2015
Dawes 1987
-
- Dawes C. Physiological factors affecting salivary flow rate, oral sugar clearance, and the sensation of dry mouth in man. Journal of Dental Research 1987;66 Spec No:648‐53. - PubMed
Egger 1997
Epstein 1994
-
- Epstein JB, Burchell JL, Emerton S, Le ND, Silverman S Jr. A clinical trial of bethanechol in patients with xerostomia after radiation therapy. A pilot study. Oral Surgery, Oral Medicine, and Oral Pathology 1994;77(6):610‐4. - PubMed
GRADE 2004
Guchelaar 1997
-
- Guchelaar HJ, Vermes A, Meerwaldt JH. Radiation‐induced xerostomia: pathophysiology, clinical course and supportive treatment. Supportive Care in Cancer 1997;5(4):281‐8. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Kontopantelis 2012
-
- Kontopantelis E, Reeves D. Performance of statistical methods for meta‐analysis when true study effects are non‐normally distributed: A simulation study. Statistical Methods in Medical Research 2012;21(4):409‐26. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Mercadante 2017
-
- Mercadante V, Al Hamad A, Lodi G, Porter S, Fedele S. Interventions for the management of radiotherapy‐induced xerostomia and hyposalivation: A systematic review and meta‐analysis. Oral Oncology 2017;66:64‐74. - PubMed
Navazesh 1992
-
- Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. Journal of Dental Research 1992;71(7):1363‐9. - PubMed
Pankhurst 1996
-
- Pankhurst CL, Smith EC, Rogers JO, Dunne SM, Jackson SH, Proctor G. Diagnosis and management of the dry mouth: Part 1. Dental Update 1996;23(2):56‐62. - PubMed
Porter 2004
-
- Porter SR, Scully C, Hegarty AM. An update of the etiology and management of xerostomia. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2004;97(1):28‐46. - PubMed
Rades 2004
-
- Rades D, Fehlauer F, Bajrovic A, Mahlmann B, Richter E, Alberti W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiotherapy and Oncology 2004;70(3):261‐4. - PubMed
Rode 2001
-
- Rode M, Smid L, Budihna M, Gassperssic D, Rode M, Soba E. The influence of pilocarpine and biperiden on pH value and calcium, phosphate, and bicarbonate concentrations in saliva during and after radiotherapy for head and neck cancer. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2001;92(5):509‐14. - PubMed
Shiboski 2007
-
- Shiboski CH, Hodgson TA, Ship JA, Schiodt M. Management of salivary hypofunction during and after radiotherapy. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontics 2007;103 Suppl(S66):e1‐19. - PubMed
Sreebny 1996
-
- Sreebny LM. Xerostomia: diagnosis, management and clinical complications. In: Edgar WM, O'Mullane DM editor(s). Saliva and Oral Health. 2nd Edition. London: British Dental Association, 1996:43‐66.
Takahashi 1986
-
- Takahashi T, Nagai K, Miyaishi K, Maehara Y, Niibe H. Clinical study of the radioprotective effects of amifostine (TM‐08310, WR‐2721) on chronic radiation injury. International Journal of Radiation Oncology, Biology, Physics 1986;12:935‐8. - PubMed
Wiseman 1995
-
- Wiseman LR, Faulds D. Oral pilocarpine: a review of its pharmacological properties and clinical potential in xerostomia. Drugs 1995;49(1):143‐55. - PubMed
Worthington 2011
Zimmerman 1997
-
- Zimmerman RP, Mark RJ, Tran LM, Juillard GF. Concomitant pilocarpine during head and neck irradiation is associated with decreased posttreatment xerostomia. International Journal of Radiation Oncology, Biology, Physics 1997;37(3):571‐5. - PubMed
References to other published versions of this review
Tavender 2004
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

