A Systematic Review of Longitudinal Studies Which Measure Alzheimer's Disease Biomarkers
- PMID: 28759968
- PMCID: PMC5611893
- DOI: 10.3233/JAD-170261
A Systematic Review of Longitudinal Studies Which Measure Alzheimer's Disease Biomarkers
Abstract
Alzheimer's disease (AD) is a progressive and fatal neurodegenerative disease, with no effective treatment or cure. A gold standard therapy would be treatment to slow or halt disease progression; however, knowledge of causation in the early stages of AD is very limited. In order to determine effective endpoints for possible therapies, a number of quantitative surrogate markers of disease progression have been suggested, including biochemical and imaging biomarkers. The dynamics of these various surrogate markers over time, particularly in relation to disease development, are, however, not well characterized. We reviewed the literature for studies that measured cerebrospinal fluid or plasma amyloid-β and tau, or took magnetic resonance image or fluorodeoxyglucose/Pittsburgh compound B-positron electron tomography scans, in longitudinal cohort studies. We summarized the properties of the major cohort studies in various countries, commonly used diagnosis methods and study designs. We have concluded that additional studies with repeat measures over time in a representative population cohort are needed to address the gap in knowledge of AD progression. Based on our analysis, we suggest directions in which research could move in order to advance our understanding of this complex disease, including repeat biomarker measurements, standardization and increased sample sizes.
Keywords: Alzheimer’s disease; biomarker; cross-sectional; dementia; longitudinal.
Figures
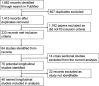
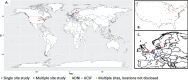
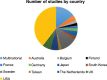
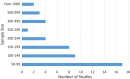
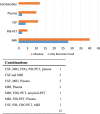
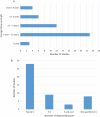
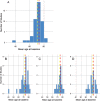
References
-
- Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP (2013) The global prevalence of dementia:A systematic review andmetaanalysis. Alzheimers Dement 9, 63–75.e62. - PubMed
-
- Small DH, Mok SS, Bornstein JC (2001) Alzheimer’s disease and Abeta toxicity: From top to bottom. Nat Rev Neurosci 2, 595–598. - PubMed
-
- Ittner LM, Gotz J (2011) Amyloid-beta and tau–a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci 12, 65–72. - PubMed
-
- Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259. - PubMed
-
- Rosen WG, Mohs RC, Davis KL (1984) A new rating scale for Alzheimer’s disease. Am J Psychiatry 141, 1356–1364. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

