Generation and characterization of new monoclonal antibodies targeting the PHF1 and AT8 epitopes on human tau
- PMID: 28760159
- PMCID: PMC5537986
- DOI: 10.1186/s40478-017-0458-0
Generation and characterization of new monoclonal antibodies targeting the PHF1 and AT8 epitopes on human tau
Abstract
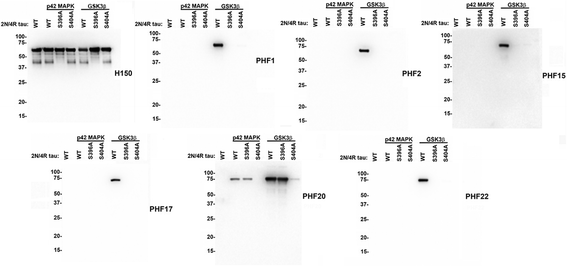
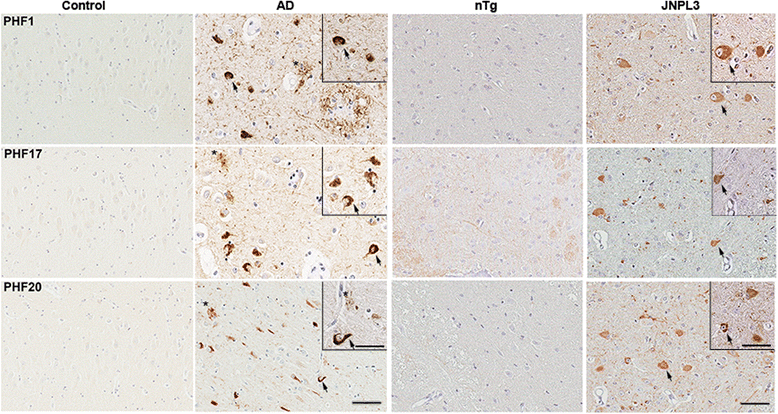
Tauopathies are a group of neurodegenerative disorders, including Alzheimer's disease, defined by the presence of brain pathological inclusions comprised of abnormally aggregated and highly phosphorylated tau protein. The abundance of brain tau aggregates correlates with disease severity and select phospho-tau epitopes increase at early stages of disease. We generated and characterized a series of novel monoclonal antibodies directed to tau phosphorylated at several of these phospho-epitopes, including Ser396/Ser404, Ser404 and Thr205. We also generated phosphorylation independent antibodies against amino acid residues 193-211. We show that most of these antibodies are highly specific for tau and strongly recognize pathological inclusions in human brains and in a transgenic mouse model of tauopathy. They also reveal epitope-specific differences in the biochemical properties of Alzheimer's disease sarkosyl-insoluble tau. These new reagents will be useful for investigating the progression of tau pathology and further as tools to target the cellular transmission of tau pathology.
Keywords: Alzheimer’s disease; Antibodies; Phosphorylation; Tau; Transgenic mice.
Conflict of interest statement
Ethics approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
All procedures performed in studies involving animals were in accordance with the ethical standards of the University of Florida.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Novel Phospho-Tau Monoclonal Antibody Generated Using a Liposomal Vaccine, with Enhanced Recognition of a Conformational Tauopathy Epitope.J Alzheimers Dis. 2017;56(2):585-599. doi: 10.3233/JAD-160695. J Alzheimers Dis. 2017. PMID: 28035925 Free PMC article.
-
Phosphorylated tau in the retina correlates with tau pathology in the brain in Alzheimer's disease and primary tauopathies.Acta Neuropathol. 2023 Feb;145(2):197-218. doi: 10.1007/s00401-022-02525-1. Epub 2022 Dec 8. Acta Neuropathol. 2023. PMID: 36480077
-
Designing antibodies against LRRK2-targeted tau epitopes.PLoS One. 2018 Sep 27;13(9):e0204367. doi: 10.1371/journal.pone.0204367. eCollection 2018. PLoS One. 2018. PMID: 30261006 Free PMC article.
-
Tau Ser208 phosphorylation promotes aggregation and reveals neuropathologic diversity in Alzheimer's disease and other tauopathies.Acta Neuropathol Commun. 2020 Jun 22;8(1):88. doi: 10.1186/s40478-020-00967-w. Acta Neuropathol Commun. 2020. PMID: 32571418 Free PMC article.
-
Chaperone-like antibodies targeting misfolded tau protein: new vistas in the immunotherapy of neurodegenerative foldopathies.J Alzheimers Dis. 2008 Oct;15(2):169-79. doi: 10.3233/jad-2008-15203. J Alzheimers Dis. 2008. PMID: 18953106 Review.
Cited by
-
Human tauopathy strains defined by phosphorylation in R1-R2 repeat domains of tau.Acta Neuropathol Commun. 2023 Oct 27;11(1):172. doi: 10.1186/s40478-023-01664-0. Acta Neuropathol Commun. 2023. PMID: 37891635 Free PMC article.
-
Novel SOD1 monoclonal antibodies against the electrostatic loop preferentially detect misfolded SOD1 aggregates.Neurosci Lett. 2021 Jan 18;742:135553. doi: 10.1016/j.neulet.2020.135553. Epub 2020 Dec 17. Neurosci Lett. 2021. PMID: 33346076 Free PMC article.
-
Soluble brain homogenates from diverse human and mouse sources preferentially seed diffuse Aβ plaque pathology when injected into newborn mouse hosts.Free Neuropathol. 2022 Jan 11;3(9):9. doi: 10.17879/freeneuropathology-2022-3766. Epub 2022 Mar 23. Free Neuropathol. 2022. PMID: 35494163 Free PMC article.
-
A novel pThr217 tau monoclonal antibody reveals neuropathological heterogeneity in tauopathies.Sci Rep. 2025 Jun 5;15(1):19865. doi: 10.1038/s41598-025-04291-y. Sci Rep. 2025. PMID: 40473739 Free PMC article.
-
Combining P301L and S320F tau variants produces a novel accelerated model of tauopathy.Hum Mol Genet. 2019 Oct 1;28(19):3255-3269. doi: 10.1093/hmg/ddz151. Hum Mol Genet. 2019. PMID: 31261380 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

