Transforming growth factor β1 enhances adhesion of endometrial cells to mesothelium by regulating integrin expression
- PMID: 28760197
- PMCID: PMC5595173
- DOI: 10.5483/bmbrep.2017.50.8.097
Transforming growth factor β1 enhances adhesion of endometrial cells to mesothelium by regulating integrin expression
Abstract
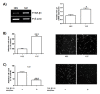
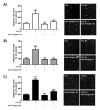
Endometriosis is the abnormal growth of endometrial cells outside the uterus, causing pelvic pain and infertility. Furthermore, adhesion of endometrial tissue fragments to pelvic mesothelium is required for the initial step of endometriosis formation outside uterus. TGF-β1 and adhesion molecules importantly function for adhesion of endometrial tissue fragments to mesothelium outside uterus. However, the function of TGF-β1 on the regulation of adhesion molecule expression for adhesion of endometrial tissue fragments to mesothelium has not been fully elucidated. Interestingly, transforming growth factor β1 (TGF-β1) expression was higher in endometriotic epithelial cells than in normal endometrial cells. The adhesion efficiency of endometriotic epithelial cells to mesothelial cells was also higher than that of normal endometrial cells. Moreover, TGF-β1 directly induced the adhesion of endometrial cells to mesothelial cells through the regulation of integrin of αV, α6, β1, and β4 via the activation of the TGF-β1/TGF-βRI/Smad2 signaling pathway. Conversely, the adhesion of TGF-β1-stimulated endometrial cells to mesothelial cells was clearly reduced following treatment with neutralizing antibodies against specific TGF-β1-mediated integrins αV, β1, and β4 on the endometrial cell membrane. Taken together, these results suggest that TGF-β1 may act to promote the initiation of endometriosis by enhancing integrin-mediated cell-cell adhesion. [BMB Reports 2017; 50(8): 429-434].
Conflict of interest statement
The authors have no conflicting financial interests.
Figures


References
-
- Bedir R, Sehitoglu I, Balik G, et al. The role of the adhesion molecule Nectin-4 in the pathogenesis of endometriosis. Clin Exp Obstet Gynecol. 2016;43:463–466. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

