Silencing repetitive DNA
- PMID: 28760198
- PMCID: PMC5538820
- DOI: 10.7554/eLife.29503
Silencing repetitive DNA
Abstract
Some RNAs in mammalian cells can help to silence the DNA they are transcribed from.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures
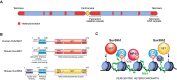
Comment on
-
Major satellite repeat RNA stabilize heterochromatin retention of Suv39h enzymes by RNA-nucleosome association and RNA:DNA hybrid formation.Elife. 2017 Aug 1;6:e25293. doi: 10.7554/eLife.25293. Elife. 2017. PMID: 28760199 Free PMC article.
-
RNA-dependent stabilization of SUV39H1 at constitutive heterochromatin.Elife. 2017 Aug 1;6:e25299. doi: 10.7554/eLife.25299. Elife. 2017. PMID: 28760200 Free PMC article.
-
Impact of nucleic acid and methylated H3K9 binding activities of Suv39h1 on its heterochromatin assembly.Elife. 2017 Aug 1;6:e25317. doi: 10.7554/eLife.25317. Elife. 2017. PMID: 28760201 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

