MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in Cardiorenal Syndrome Type 4
- PMID: 28760335
- PMCID: PMC5787401
- DOI: 10.1016/j.kint.2017.05.014
MicroRNA-21 regulates peroxisome proliferator-activated receptor alpha, a molecular mechanism of cardiac pathology in Cardiorenal Syndrome Type 4
Abstract
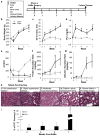
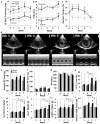
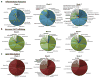
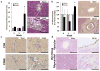
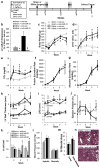
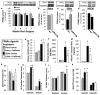
Cardiovascular events are the leading cause of death in patients with chronic kidney disease (CKD), although the pathological mechanisms are poorly understood. Here we longitudinally characterized left ventricle pathology in a 5/6 nephrectomy rat model of CKD and identify novel molecular mediators. Next-generation sequencing of left ventricle mRNA and microRNA (miRNA) was performed at physiologically distinct points in disease progression, identifying alterations in genes in numerous immune, lipid metabolism, and inflammatory pathways, as well as several miRNAs. MiRNA miR-21-5p was increased in our dataset and has been reported to regulate many identified pathways. Suppression of miR-21-5p protected rats with 5/6 nephrectomy from developing left ventricle hypertrophy and improved left ventricle function. Next-generation mRNA sequencing revealed that miR-21-5p suppression altered gene expression in peroxisome proliferator-activated receptor alpha (PPARα) regulated pathways in the left ventricle. PPARα, a miR-21-5p target, is the primary PPAR isoform in the heart, importantly involved in regulating fatty acid metabolism. Therapeutic delivery of low-dose PPARα agonist (clofibrate) to rats with 5/6 nephrectomy improved cardiac function and prevented left ventricle dilation. Thus, comprehensive characterization of left ventricle molecular changes highlights the involvement of numerous signaling pathways not previously explored in CKD models and identified PPARα as a potential therapeutic target for CKD-related cardiac dysfunction.
Keywords: cardiovascular disease; chronic kidney disease; inflammation; renal pathology.
Copyright © 2017 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–S119. - PubMed
-
- Owan TE, Hodge DO, Herges, et al. Secular trends in renal dysfunction and outcomes in hospitalized heart failure patients. J Card Fail. 2006;12:257–262. - PubMed
-
- Krumholz HM, Chen YT, Vaccarino V, et al. Correlates and impact on outcomes of worsening renal function in patients > or ¼65 years of age with heart failure. Am J Cardiol. 2000;85:1110–1113. - PubMed
-
- Nohria A, Hasselblad V, Stebbins A, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–1274. - PubMed
-
- Butler J, Forman DE, Abraham WT, et al. Relationship between heart failure treatment and development of worsening renal function among hospitalized patients. Am Heart J. 2004;147:331–338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

