Demographic and genetic consequences of disturbed sex determination
- PMID: 28760767
- PMCID: PMC5540866
- DOI: 10.1098/rstb.2016.0326
Demographic and genetic consequences of disturbed sex determination
Abstract
During sex determination, genetic and/or environmental factors determine the cascade of processes of gonad development. Many organisms, therefore, have a developmental window in which their sex determination can be sensitive to, for example, unusual temperatures or chemical pollutants. Disturbed environments can distort population sex ratios and may even cause sex reversal in species with genetic sex determination. The resulting genotype-phenotype mismatches can have long-lasting effects on population demography and genetics. I review the theoretical and empirical work in this context and explore in a simple population model the role of the fitness vyy of chromosomally aberrant YY genotypes that are a consequence of environmentally induced feminization. Low vyy is mostly beneficial for population growth. During feminization, low vyy reduces the proportion of genetic males and hence accelerates population growth, especially at low rates of feminization and at high fitness costs of the feminization itself (i.e. when feminization would otherwise not affect population dynamics much). When sex reversal ceases, low vyy mitigates the negative effects of feminization and can even prevent population extinction. Little is known about vyy in natural populations. The available models now need to be parametrized in order to better predict the long-term consequences of disturbed sex determination.This article is part of the themed issue 'Adult sex ratios and reproductive decisions: a critical re-examination of sex differences in human and animal societies'.
Keywords: climate change; endocrine-disrupting chemicals; environmental sex reversal; extinction; population growth; sex determination.
© 2017 The Author(s).
Conflict of interest statement
I declare I have no competing interests.
Figures
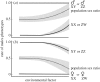
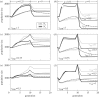

References
-
- Beukeboom LW, Perrin N. 2014. The evolution of sex determination, 1–222 p Oxford, UK: Oxford University Press.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
