Improving cartilage phenotype from differentiated pericytes in tunable peptide hydrogels
- PMID: 28761049
- PMCID: PMC5537289
- DOI: 10.1038/s41598-017-07255-z
Improving cartilage phenotype from differentiated pericytes in tunable peptide hydrogels
Abstract
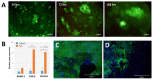
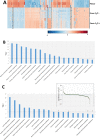
Differentiation of stem cells to chondrocytes in vitro usually results in a heterogeneous phenotype. This is evident in the often detected over expression of type X collagen which, in hyaline cartilage structure is not characteristic of the mid-zone but of the deep-zone ossifying tissue. Methods to better match cartilage developed in vitro to characteristic in vivo features are therefore highly desirable in regenerative medicine. This study compares phenotype characteristics between pericytes, obtained from human adipose tissue, differentiated using diphenylalanine/serine (F2/S) peptide hydrogels with the more widely used chemical induced method for chondrogenesis. Significantly higher levels of type II collagen were noted when pericytes undergo chondrogenesis in the hydrogel in the absence of induction media. There is also a balanced expression of collagen relative to aggrecan production, a feature which was biased toward collagen production when cells were cultured with induction media. Lastly, metabolic profiles of each system show considerable overlap between both differentiation methods but subtle differences which potentially give rise to their resultant phenotype can be ascertained. The study highlights how material and chemical alterations in the cellular microenvironment have wide ranging effects on resultant tissue type.
Conflict of interest statement
RVU is CSO of Biogelx, a spinout company that markets the gels developed and used in this paper.
Figures



References
-
- Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4397–4402. doi: 10.1073/pnas.052716199. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

