Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice
- PMID: 28761067
- PMCID: PMC5537224
- DOI: 10.1038/s41467-017-00178-3
Every-other-day feeding extends lifespan but fails to delay many symptoms of aging in mice
Abstract
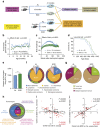
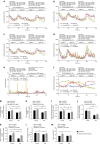
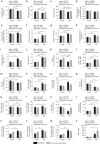
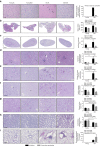
Dietary restriction regimes extend lifespan in various animal models. Here we show that longevity in male C57BL/6J mice subjected to every-other-day feeding is associated with a delayed onset of neoplastic disease that naturally limits lifespan in these animals. We compare more than 200 phenotypes in over 20 tissues in aged animals fed with a lifelong every-other-day feeding or ad libitum access to food diet to determine whether molecular, cellular, physiological and histopathological aging features develop more slowly in every-other-day feeding mice than in controls. We also analyze the effects of every-other-day feeding on young mice on shorter-term every-other-day feeding or ad libitum to account for possible aging-independent restriction effects. Our large-scale analysis reveals overall only limited evidence for a retardation of the aging rate in every-other-day feeding mice. The data indicate that every-other-day feeding-induced longevity is sufficiently explained by delays in life-limiting neoplastic disorders and is not associated with a more general slowing of the aging process in mice.Dietary restriction can extend the life of various model organisms. Here, Xie et al. show that intermittent periods of fasting achieved through every-other-day feeding protect mice against neoplastic disease but do not broadly delay organismal aging in animals.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Miller, R. A. in Hazzard’s Geriatric Medicine and Gerontology (eds Halter, J. B., Ouslander, J. G., Tinetti, M. E., Studenski, S., High, K. P. & Asthana S.) (The McGraw-Hill Companies, Inc., 2009).
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

